Cost-effectiveness Analysis of Genetic Testing and Tailored First-Line Therapy for Patients With Metastatic Gastrointestinal Stromal Tumors
- PMID: 32986105
- PMCID: PMC7522695
- DOI: 10.1001/jamanetworkopen.2020.13565
Cost-effectiveness Analysis of Genetic Testing and Tailored First-Line Therapy for Patients With Metastatic Gastrointestinal Stromal Tumors
Abstract
Importance: Gastrointestinal stromal tumor (GIST) is frequently driven by oncogenic KIT variations. Imatinib targeting of KIT marked a new era in GIST treatment and ushered in precision oncological treatment for all solid malignant neoplasms. However, studies on the molecular biological traits of GIST have found that tumors respond differentially to imatinib dosage based on the KIT exon with variation. Despite this knowledge, few patients undergo genetic testing at diagnosis, and empirical imatinib therapy remains routine. Barriers to genetic profiling include concerns about the cost and utility of testing.
Objective: To determine whether targeted gene testing (TGT) is a cost-effective diagnostic for patients with metastatic GIST from the US payer perspective.
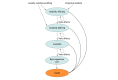
Design, setting, and participants: This economic evaluation developed a Markov model to compare the cost-effectiveness of TGT and tailored first-line therapy compared with empirical imatinib therapy among patients with a new diagnosis of metastatic GIST. The main health outcome, quality-adjusted life years (QALYs), and costs were obtained from the literature, and transitional probabilities were modeled from disease progression and survival estimates from randomized clinical trials of patients with metastatic GIST. Data analyses were conducted October 2019 to January 2020.
Exposure: TGT and tailored first-line therapy.
Main outcomes and measures: The primary outcome was QALYs and cost. Cost-effectiveness was defined using an incremental cost-effectiveness ratio, with an incremental cost-effectiveness ratio less than $100 000/QALY considered cost-effective. One-way and probabilistic sensitivity analyses were conducted to assess model stability.
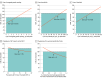
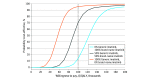
Results: Therapy directed by TGT was associated with an increase of 0.10 QALYs at a cost of $9513 compared with the empirical imatinib approach, leading to an incremental cost-effectiveness ratio of $92 100. These findings were sensitive to the costs of TGT, drugs, and health utility model inputs. Therapy directed by TGT remained cost-effective for genetic testing costs up to $3730. Probabilistic sensitivity analysis found that TGT-directed therapy was considered cost-effective 70% of the time.
Conclusions and relevance: These findings suggest that using genetic testing to match treatment of KIT variations to imatinib dosing is a cost-effective approach compared with empirical imatinib.
Conflict of interest statement
Figures
Similar articles
-
Budgetary impact of treatment with adjuvant imatinib for 1 year following surgical resection of Kit-positive localized gastrointestinal stromal tumors.J Manag Care Pharm. 2010 Sep;16(7):482-91. doi: 10.18553/jmcp.2010.16.7.482. J Manag Care Pharm. 2010. PMID: 20726677 Free PMC article.
-
Optimizing the dose in patients treated with imatinib as first line treatment for gastrointestinal stromal tumours: A cost-effectiveness study.Br J Clin Pharmacol. 2019 Sep;85(9):1994-2001. doi: 10.1111/bcp.13990. Epub 2019 Jul 17. Br J Clin Pharmacol. 2019. PMID: 31112617 Free PMC article.
-
Cost-effectiveness of 3-years of adjuvant imatinib in gastrointestinal stromal tumors (GIST) in the United States.J Med Econ. 2013;16(1):150-9. doi: 10.3111/13696998.2012.709204. Epub 2012 Jul 19. J Med Econ. 2013. PMID: 22762291
-
Cost effectiveness of tyrosine kinase inhibitor therapy in metastatic gastrointestinal stromal tumors.J Med Econ. 2010;13(4):681-90. doi: 10.3111/13696998.2010.534670. Epub 2010 Nov 10. J Med Econ. 2010. PMID: 21067355 Review.
-
Imatinib as adjuvant treatment following resection of KIT-positive gastrointestinal stromal tumours.Health Technol Assess. 2010 Oct;14(Suppl. 2):63-70. doi: 10.3310/hta14suppl2/09. Health Technol Assess. 2010. PMID: 21047493 Review.
Cited by
-
Insights into the medical management of gastrointestinal stromal tumours: lessons learnt from a dedicated gastrointestinal stromal tumour clinic in North India.Ecancermedicalscience. 2023 Jan 16;17:1497. doi: 10.3332/ecancer.2023.1497. eCollection 2023. Ecancermedicalscience. 2023. PMID: 36816783 Free PMC article.
-
Gastrointestinal Stromal Tumor: New Insights for a Multimodal Approach.Surg Oncol Clin N Am. 2022 Jul;31(3):431-446. doi: 10.1016/j.soc.2022.03.007. Epub 2022 May 31. Surg Oncol Clin N Am. 2022. PMID: 35715143 Free PMC article. Review.
-
Nationwide evaluation of mutation-tailored treatment of gastrointestinal stromal tumors in daily clinical practice.Gastric Cancer. 2021 Sep;24(5):990-1002. doi: 10.1007/s10120-021-01190-9. Epub 2021 Apr 28. Gastric Cancer. 2021. PMID: 33909171 Free PMC article.
-
Cost-Effectiveness Analysis of Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumor: A Systematic Review.Front Public Health. 2022 Jan 10;9:768765. doi: 10.3389/fpubh.2021.768765. eCollection 2021. Front Public Health. 2022. PMID: 35083189 Free PMC article.
-
Consensus Statements on Precision Oncology in the China Greater Bay Area.JCO Precis Oncol. 2023 Jun;7:e2200649. doi: 10.1200/PO.22.00649. JCO Precis Oncol. 2023. PMID: 37315266 Free PMC article.
References
-
- Demetri GD, Reichardt P, Kang YK, et al. ; GRID study investigators . Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295-302. doi:10.1016/S0140-6736(12)61857-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

