Safety and effectiveness of pirfenidone combined with carboplatin-based chemotherapy in patients with idiopathic pulmonary fibrosis and non-small cell lung cancer: A retrospective cohort study
- PMID: 32986306
- PMCID: PMC7606001
- DOI: 10.1111/1759-7714.13675
Safety and effectiveness of pirfenidone combined with carboplatin-based chemotherapy in patients with idiopathic pulmonary fibrosis and non-small cell lung cancer: A retrospective cohort study
Abstract
Background: Pirfenidone is an antifibrotic agent that is potentially effective for the treatment of idiopathic pulmonary fibrosis (IPF). However, no study has reported on its prophylactic value against chemotherapy-associated acute IPF exacerbations when combined with chemotherapy for non-small cell lung cancer (NSCLC). The present study assessed the safety and effectiveness of pirfenidone combined with carboplatin-based chemotherapy or immune checkpoint inhibitors (ICIs) in patients with IPF and NSCLC.
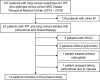
Methods: A total of 14 patients with IPF and NSCLC who received treatment from 2013 to 2019 were included. Patients were treated with pirfenidone combined with carboplatin and nanoparticle albumin-bound paclitaxel or S-1 as first-line chemotherapy. After confirming disease progression, patients received cytotoxic agents or ICIs, including nivolumab and pembrolizumab. Pirfenidone was continued regardless of chemotherapy changes. Overall survival (OS) and progression-free survival (PFS) for lung cancer and IPF were calculated. Moreover, the cumulative incidence of acute exacerbation of IPF (AE-IPF) within one year was evaluated.
Results: Median PFS for lung cancer was 110 days (95% confidence interval [CI]: 57-199 days), while the median OS was 362 days (95% CI: 220-526 days). Moreover, PFS for IPF was 447 days (95% CI: 286-indeterminate days), and the cumulative incidence of AE-IPF within one year was 18%. Notably, none of the patients developed AE-IPF associated with first-line chemotherapy. Among the included patients, four received ICIs, none of whom developed ICI-associated AE-IPF.
Conclusions: The present study found that pirfenidone combined with carboplatin-based regimens or ICIs might be safe first-line chemotherapy for patients with IPF and NSCLC.
Key points: SIGNIFICANT FINDINGS OF THE STUDY: No patients with IPF and NSCLC who received pirfenidone in combination with first-line carboplatin-based chemotherapy or late-line ICIs developed acute IPF exacerbations. What this study adds Pirfenidone might have a prophylactic effect against chemotherapy-associated AE-IPF.
Keywords: Acute exacerbation; immune checkpoint inhibitors; interstitial pneumonia; platinum-based chemotherapy; toxicity.
© 2020 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures


Similar articles
-
A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study).Respir Res. 2016 Jul 22;17(1):90. doi: 10.1186/s12931-016-0398-4. Respir Res. 2016. PMID: 27450274 Free PMC article. Clinical Trial.
-
Real-life experiences in a single center: efficacy of pirfenidone in idiopathic pulmonary fibrosis and fibrotic idiopathic non-specific interstitial pneumonia patients.Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620963015. doi: 10.1177/1753466620963015. Ther Adv Respir Dis. 2020. PMID: 33070705 Free PMC article.
-
Treatment Rationale and Design for J-SONIC: A Randomized Study of Carboplatin Plus Nab-paclitaxel With or Without Nintedanib for Advanced Non-Small-cell Lung Cancer With Idiopathic Pulmonary Fibrosis.Clin Lung Cancer. 2018 Jan;19(1):e5-e9. doi: 10.1016/j.cllc.2017.06.003. Epub 2017 Jun 20. Clin Lung Cancer. 2018. PMID: 28687482
-
Small Cell Lung Cancer in the Course of Idiopathic Pulmonary Fibrosis-Case Report and Literature Review.Curr Oncol. 2022 Jul 18;29(7):5077-5083. doi: 10.3390/curroncol29070401. Curr Oncol. 2022. PMID: 35877261 Free PMC article. Review.
-
Clinical outcomes of non-small cell lung cancer patients with leptomeningeal metastases after immune checkpoint inhibitor treatments.Eur J Cancer. 2021 Jun;150:23-30. doi: 10.1016/j.ejca.2021.03.037. Epub 2021 Apr 18. Eur J Cancer. 2021. PMID: 33882375
Cited by
-
Lung cancer in patients with fibrosing interstitial lung diseases: an overview of current knowledge and challenges.ERJ Open Res. 2022 Jun 20;8(2):00115-2022. doi: 10.1183/23120541.00115-2022. eCollection 2022 Apr. ERJ Open Res. 2022. PMID: 35747227 Free PMC article. Review.
-
Risk factors and management of lung cancer in idiopathic pulmonary fibrosis: A comprehensive review.Sarcoidosis Vasc Diffuse Lung Dis. 2025 Mar 18;42(1):15604. doi: 10.36141/svdld.v42i1.15604. Sarcoidosis Vasc Diffuse Lung Dis. 2025. PMID: 40100103 Free PMC article.
-
Acute exacerbation of postoperative idiopathic pulmonary fibrosis in a patient with lung cancer caused by invasive mechanical ventilation: A case report.Heliyon. 2023 Oct 31;9(11):e21538. doi: 10.1016/j.heliyon.2023.e21538. eCollection 2023 Nov. Heliyon. 2023. PMID: 38027643 Free PMC article.
-
[Pirfenidone inhibits bladder cancer xenograft growth in mice by regulating regulatory T cells].Nan Fang Yi Ke Da Xue Xue Bao. 2025 Jul 20;45(7):1513-1518. doi: 10.12122/j.issn.1673-4254.2025.07.18. Nan Fang Yi Ke Da Xue Xue Bao. 2025. PMID: 40673314 Free PMC article. Chinese.
-
The unexplored mechanism of antitumoral effect of pirfenidone in melanoma cells.Sci Rep. 2025 Aug 1;15(1):28071. doi: 10.1038/s41598-025-13584-1. Sci Rep. 2025. PMID: 40751072 Free PMC article.
References
-
- American Thoracic Society . Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000; 161: 646–64. - PubMed
-
- Wells AU, Desai SR, Rubens MB et al Idiopathic pulmonary fibrosis: A composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003; 167: 962–9. - PubMed
-
- du Bois RM, Weycker D, Albera C et al Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 184: 459–66. - PubMed
-
- Ley B, Ryerson CJ, Vittinghoff E et al A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012; 156: 684–91. - PubMed
-
- du Bois RM, Weycker D, Albera C et al Forced vital capacity in patients with idiopathic pulmonary fibrosis: Test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011; 184: 1382–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

