Safety and effectiveness of pirfenidone combined with carboplatin-based chemotherapy in patients with idiopathic pulmonary fibrosis and non-small cell lung cancer: A retrospective cohort study
- PMID: 32986306
- PMCID: PMC7606001
- DOI: 10.1111/1759-7714.13675
Safety and effectiveness of pirfenidone combined with carboplatin-based chemotherapy in patients with idiopathic pulmonary fibrosis and non-small cell lung cancer: A retrospective cohort study
Abstract
Background: Pirfenidone is an antifibrotic agent that is potentially effective for the treatment of idiopathic pulmonary fibrosis (IPF). However, no study has reported on its prophylactic value against chemotherapy-associated acute IPF exacerbations when combined with chemotherapy for non-small cell lung cancer (NSCLC). The present study assessed the safety and effectiveness of pirfenidone combined with carboplatin-based chemotherapy or immune checkpoint inhibitors (ICIs) in patients with IPF and NSCLC.
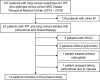
Methods: A total of 14 patients with IPF and NSCLC who received treatment from 2013 to 2019 were included. Patients were treated with pirfenidone combined with carboplatin and nanoparticle albumin-bound paclitaxel or S-1 as first-line chemotherapy. After confirming disease progression, patients received cytotoxic agents or ICIs, including nivolumab and pembrolizumab. Pirfenidone was continued regardless of chemotherapy changes. Overall survival (OS) and progression-free survival (PFS) for lung cancer and IPF were calculated. Moreover, the cumulative incidence of acute exacerbation of IPF (AE-IPF) within one year was evaluated.
Results: Median PFS for lung cancer was 110 days (95% confidence interval [CI]: 57-199 days), while the median OS was 362 days (95% CI: 220-526 days). Moreover, PFS for IPF was 447 days (95% CI: 286-indeterminate days), and the cumulative incidence of AE-IPF within one year was 18%. Notably, none of the patients developed AE-IPF associated with first-line chemotherapy. Among the included patients, four received ICIs, none of whom developed ICI-associated AE-IPF.
Conclusions: The present study found that pirfenidone combined with carboplatin-based regimens or ICIs might be safe first-line chemotherapy for patients with IPF and NSCLC.
Key points: SIGNIFICANT FINDINGS OF THE STUDY: No patients with IPF and NSCLC who received pirfenidone in combination with first-line carboplatin-based chemotherapy or late-line ICIs developed acute IPF exacerbations. What this study adds Pirfenidone might have a prophylactic effect against chemotherapy-associated AE-IPF.
Keywords: Acute exacerbation; immune checkpoint inhibitors; interstitial pneumonia; platinum-based chemotherapy; toxicity.
© 2020 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures


References
-
- American Thoracic Society . Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000; 161: 646–64. - PubMed
-
- Wells AU, Desai SR, Rubens MB et al Idiopathic pulmonary fibrosis: A composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003; 167: 962–9. - PubMed
-
- du Bois RM, Weycker D, Albera C et al Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 184: 459–66. - PubMed
-
- Ley B, Ryerson CJ, Vittinghoff E et al A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012; 156: 684–91. - PubMed
-
- du Bois RM, Weycker D, Albera C et al Forced vital capacity in patients with idiopathic pulmonary fibrosis: Test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011; 184: 1382–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

