6-Prenylnaringenin from Hops Disrupts ERα-Mediated Downregulation of CYP1A1 to Facilitate Estrogen Detoxification
- PMID: 32986415
- PMCID: PMC8349113
- DOI: 10.1021/acs.chemrestox.0c00194
6-Prenylnaringenin from Hops Disrupts ERα-Mediated Downregulation of CYP1A1 to Facilitate Estrogen Detoxification
Abstract
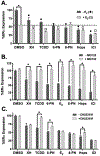
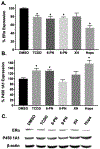
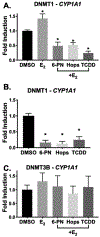
Botanical dietary supplements (BDS) containing hops are sold as women's health supplements due to the potent hop phytoestrogen, 8-prenylnaringenin (8-PN), and the cytoprotective chalcone, xanthohumol. Previous studies have shown a standardized hop extract to beneficially influence chemical estrogen carcinogenesis in vitro by fostering detoxified 2-hydroxylation over genotoxic 4-hydroxylation estrogen metabolism. In this study, hop extract and its bioactive compounds were investigated for its mechanism of action within the chemical estrogen carcinogenesis pathway, which is mainly mediated through the 4-hydroxylation pathway catalyzed by CYP1B1 that can form gentoxic quinones. Aryl hydrocarbon receptor (AhR) agonists induce CYP1A1 and CYP1B1, while estrogen receptor alpha (ERα) inhibits transcription of CYP1A1, the enzyme responsible for 2-hydroxylated estrogens and the estrogen detoxification pathway. An In-Cell Western MCF-7 cell assay revealed hop extract and 6-prenylnaringenin (6-PN) degraded ERα via an AhR-dependent mechanism. Reverse transcription PCR and xenobiotic response element luciferase assays showed hop extract and 6-PN-mediated activation of AhR and induction of CYP1A1. A reduction in estrogen-mediated DNA (cytosine-5)-methyltransferase 1 (DNMT1) downregulation of CYP1A1 accompanied this activity in a chromatin immunoprecipitation assay. Ultimately, hop extract and 6-PN induced preferential metabolism of estrogens to their detoxified form in vitro. These results suggest that the standardized hop extract and 6-PN activate AhR to attenuate epigenetic inhibition of CYP1A1 through degradation of ERα, ultimately increasing 2-hydroxylated estrogens. A new mechanism of action rationalizes the positive influence of hop BDS and 6-PN on oxidative estrogen metabolism in vitro and, thus, potentially on chemical estrogen carcinogenesis. The findings underscore the importance of elucidating various biological mechanisms of action and standardizing BDS to multiple phytoconstituents for optimal resilience promoting properties.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Hop (Humulus lupulus L.) Extract and 6-Prenylnaringenin Induce P450 1A1 Catalyzed Estrogen 2-Hydroxylation.Chem Res Toxicol. 2016 Jul 18;29(7):1142-50. doi: 10.1021/acs.chemrestox.6b00112. Epub 2016 Jun 22. Chem Res Toxicol. 2016. PMID: 27269377 Free PMC article.
-
The Multiple Biological Targets of Hops and Bioactive Compounds.Chem Res Toxicol. 2019 Feb 18;32(2):222-233. doi: 10.1021/acs.chemrestox.8b00345. Epub 2019 Jan 22. Chem Res Toxicol. 2019. PMID: 30608650 Free PMC article. Review.
-
AHR agonistic effects of 6-PN contribute to potential beneficial effects of Hops extract.Mol Cell Endocrinol. 2022 Mar 1;543:111540. doi: 10.1016/j.mce.2021.111540. Epub 2021 Dec 26. Mol Cell Endocrinol. 2022. PMID: 34965452
-
Red Clover Aryl Hydrocarbon Receptor (AhR) and Estrogen Receptor (ER) Agonists Enhance Genotoxic Estrogen Metabolism.Chem Res Toxicol. 2017 Nov 20;30(11):2084-2092. doi: 10.1021/acs.chemrestox.7b00237. Epub 2017 Oct 19. Chem Res Toxicol. 2017. PMID: 28985473 Free PMC article.
-
Flavonoids as Phytoestrogenic Components of Hops and Beer.Molecules. 2020 Sep 14;25(18):4201. doi: 10.3390/molecules25184201. Molecules. 2020. PMID: 32937790 Free PMC article. Review.
Cited by
-
DESIGNER fraction concept unmasks minor bioactive constituents in red clover (Trifolium pratense L.).Phytochemistry. 2023 Oct;214:113789. doi: 10.1016/j.phytochem.2023.113789. Epub 2023 Jul 21. Phytochemistry. 2023. PMID: 37482264 Free PMC article.
-
Sex disparities in hepatocellular carcinoma immunotherapy: hormonal and genetic influences on treatment efficacy.Front Immunol. 2025 May 14;16:1607374. doi: 10.3389/fimmu.2025.1607374. eCollection 2025. Front Immunol. 2025. PMID: 40438106 Free PMC article. Review.
-
Humulus lupulus (Hop)-Derived Chemical Compounds Present Antiproliferative Activity on Various Cancer Cell Types: A Meta-Regression Based Panoramic Meta-Analysis.Pharmaceuticals (Basel). 2025 Jul 31;18(8):1139. doi: 10.3390/ph18081139. Pharmaceuticals (Basel). 2025. PMID: 40872530 Free PMC article. Review.
-
Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health.Antioxidants (Basel). 2021 Nov 26;10(12):1893. doi: 10.3390/antiox10121893. Antioxidants (Basel). 2021. PMID: 34942997 Free PMC article. Review.
References
-
- Smith KKT, Eckl V, Morton C, Stredney R,, Herbal Supplement Sales in US Increased 8.5% in 2017, Topping $8 Billion. HerbalGram, The Jounral of the American Botanical Council 2018, 62–71.
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing I Group for the Women's Health Initiative, Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. - PubMed
-
- van Breemen RB, Yuan Y, Banuvar S, Shulman LP, Qiu X, Alvarenga RF, Chen SN, Dietz BM, Bolton JL, Pauli GF, Krause E, Viana M, Nikolic D, Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol Nutr Food Res 2014, 58, 1962–1969. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

