Programmed DNA Elimination in Vertebrates
- PMID: 32986476
- PMCID: PMC8715500
- DOI: 10.1146/annurev-animal-061220-023220
Programmed DNA Elimination in Vertebrates
Abstract
Over the last few decades, an increasing number of vertebrate taxa have been identified that undergo programmed genome rearrangement, or programmed DNA loss, during development. In these organisms, the genome of germ cells is often reproducibly different from the genome of all other cells within the body. Although we clearly have not identified all vertebrate taxa that undergo programmed genome loss, the list of species known to undergo loss now represents ∼10% of vertebrate species, including several basally diverging lineages. Recent studies have shed new light on the targets and mechanisms of DNA loss and their association with canonical modes of DNA silencing. Ultimately, expansion of these studies into a larger collection of taxa will aid in reconstructing patterns of shared/independent ancestry of programmed DNA loss in the vertebrate lineage, as well as more recent evolutionary events that have shaped the structure and content of eliminated DNA.
Keywords: evolution; genome; programmed DNA loss; programmed genome rearrangement; vertebrate.
Figures
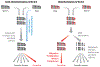
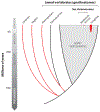



References
-
- Hirano M, Das S, Guo P, Cooper MD. 2011. The evolution of adaptive immunity in vertebrates. Adv. Immunol 109:125–57 - PubMed
-
- Boveri T. 1887. Über differenzierung der zellkerne während der furchung des eies von Ascaris megalocephala. Anat. Anz 2:288–693
-
- Bütschli O. 1876. Studien über die ersten Entwicklungsvorgänge der und die Konjugation der Infusorien. Abh. Senckenberg. Naturforschenden Ges 10:1–250
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
