COVID-19 Severity and Outcomes in Patients With Cancer: A Matched Cohort Study
- PMID: 32986528
- PMCID: PMC7676890
- DOI: 10.1200/JCO.20.01580
COVID-19 Severity and Outcomes in Patients With Cancer: A Matched Cohort Study
Abstract
Purpose: SARS-CoV-2 (COVID-19) is a systemic infection. Patients with cancer are immunocompromised and may be vulnerable to COVID-related morbidity and mortality. The objectives of this study were to determine if patients with cancer have worse outcomes compared with patients without cancer and to identify demographic and clinical predictors of morbidity and mortality among patients with cancer.
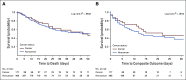
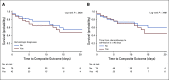
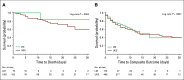
Methods: We used data from adult patients who tested positive for COVID-19 and were admitted to two New York-Presbyterian hospitals between March 3 and May 15, 2020. Patients with cancer were matched 1:4 to controls without cancer in terms of age, sex, and number of comorbidities. Using Kaplan-Meier curves and the log-rank test, we compared morbidity (intensive care unit admission and intubation) and mortality outcomes between patients with cancer and controls. Among those with cancer, we identified demographic and clinical predictors of worse outcomes using Cox proportional hazard models.
Results: We included 585 patients who were COVID-19 positive, of whom 117 had active malignancy, defined as those receiving cancer-directed therapy or under active surveillance within 6 months of admission. Presenting symptoms and in-hospital complications were similar between the cancer and noncancer groups. Nearly one half of patients with cancer were receiving therapy, and 45% of patients received cytotoxic or immunosuppressive treatment within 90 days of admission. There were no statistically significant differences in morbidity or mortality (P = .894) between patients with and without cancer.
Conclusion: We observed that patients with COVID-19 and cancer had similar outcomes compared with matched patients without cancer. This finding suggests that a diagnosis of active cancer alone and recent anticancer therapy do not predict worse COVID-19 outcomes and therefore, recommendations to limit cancer-directed therapy must be considered carefully in relation to cancer-specific outcomes and death.
Figures
Comment in
-
Reply to K. de Joode et al.J Clin Oncol. 2021 Mar 20;39(9):1093-1094. doi: 10.1200/JCO.20.03530. Epub 2021 Jan 26. J Clin Oncol. 2021. PMID: 33497249 No abstract available.
-
Remarkable Healthy Cohort of Patients With Cancer.J Clin Oncol. 2021 Mar 20;39(9):1092-1093. doi: 10.1200/JCO.20.03283. Epub 2021 Jan 26. J Clin Oncol. 2021. PMID: 33497269 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

