Characteristics of Patients With Antiphospholipid Antibody Positivity in the APS ACTION International Clinical Database and Repository
- PMID: 32986935
- PMCID: PMC10725727
- DOI: 10.1002/acr.24468
Characteristics of Patients With Antiphospholipid Antibody Positivity in the APS ACTION International Clinical Database and Repository
Abstract
Objective: To describe the baseline characteristics of patients with positivity for antiphospholipid antibodies (aPLs) who were enrolled in an international registry, the Antiphospholipid Syndrome (APS) Alliance for Clinical Trials and International Networking (APS ACTION) clinical database and repository, overall and by clinical and laboratory subtypes.
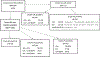
Methods: The APS ACTION registry includes adults who persistently had positivity for aPLs. We evaluated baseline sociodemographic and aPL-related (APS classification criteria and "non-criteria") characteristics of patients overall and in subgroups (aPL-positive without APS, APS overall, thrombotic APS only, obstetric APS only, and both thrombotic APS/obstetric APS). We assessed baseline characteristics of patients tested for the presence of three aPLs (lupus anticoagulant [LAC] test, anticardiolipin antibody [aCL], and anti-β2 -glycoprotein I [anti-β2 GPI]) antibodies by aPL profiles (LAC only, single, double, and triple aPL positivity).
Results: The 804 aPL-positive patients assessed in the present study had a mean age of 45 ± 13 years, were 74% female, and 68% White; additionally, 36% had other systemic autoimmune diseases. Of these 804 aPL-positive patients, 80% were classified as having APS (with 55% having thrombotic APS, 9% obstetric APS, and 15% thrombotic APS/obstetric APS). In the overall cohort, 71% had vascular thrombosis, 50% with a history of pregnancy had obstetric morbidity, and 56% had experienced at least one non-criteria manifestation. Among those with three aPLs tested (n = 660), 42% were triple aPL-positive. While single-, double-, and triple aPL-positive subgroups had similar frequencies of vascular, obstetric, and non-criteria events, these events were lowest in the single aPL subgroup, which consisted of aCLs or anti-β2 GPI only.
Conclusion: Our study demonstrates the heterogeneity of aPL-related clinical manifestations and laboratory profiles in a multicenter international cohort. Within single aPL positivity, LAC may be a major contributor to clinical events. Future prospective analyses, using standardized core laboratory aPL tests, will help clarify aPL risk profiles and improve risk stratification.
© 2020, American College of Rheumatology.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures
References
-
- Miyakis S, Lockshin M, Atsumi T, Branch D, Brey R, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. - PubMed
-
- Barbhaiya M, Andrade D, Bertolaccini M, Erkan D. Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION). In: Erkan D, Lockshin MD, editors. Antiphospholipid Syndrome: Current Research Highlights and Clinical Insights. Cham: Springer International Publishing; 2017. p. 267–76.
-
- Levine J, Branch, DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346: 752–63. - PubMed
-
- Cervera R, Serrano R, Pons-Estel G, Ceberio-Hualde L, Shoenfeld Y, Ramón E, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2014; 74: 1011–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

