Acute intermittent hypoxia boosts spinal plasticity in humans with tetraplegia
- PMID: 32987000
- PMCID: PMC9208274
- DOI: 10.1016/j.expneurol.2020.113483
Acute intermittent hypoxia boosts spinal plasticity in humans with tetraplegia
Abstract
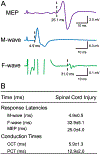
Paired corticospinal-motoneuronal stimulation (PCMS) elicits spinal synaptic plasticity in humans with chronic incomplete cervical spinal cord injury (SCI). Here, we examined whether PCMS-induced plasticity could be potentiated by acute intermittent hypoxia (AIH), a treatment also known to induce spinal synaptic plasticity in humans with chronic incomplete cervical SCI. During PCMS, we used 180 pairs of stimuli where corticospinal volleys evoked by transcranial magnetic stimulation over the hand representation of the primary motor cortex were timed to arrive at corticospinal-motoneuronal synapses of the first dorsal interosseous (FDI) muscle ~1-2 ms before the arrival of antidromic potentials elicited in motoneurons by electrical stimulation of the ulnar nerve. During AIH, participants were exposed to brief alternating episodes of hypoxic inspired gas (1 min episodes of 9% O2) and room air (1 min episodes of 20.9% O2). We examined corticospinal function by measuring motor evoked potentials (MEPs) elicited by cortical and subcortical stimulation of corticospinal axons and voluntary motor output in the FDI muscle before and after 30 min of PCMS combined with AIH (PCMS+AIH) or sham AIH (PCMS+sham-AIH). The amplitude of MEPs evoked by magnetic and electrical stimulation increased after both protocols, but most after PCMS+AIH, consistent with the hypothesis that their combined effects arise from spinal plasticity. Both protocols increased electromyographic activity in the FDI muscle to a similar extent. Thus, PCMS effects on spinal synapses of hand motoneurons can be potentiated by AIH. The possibility of different thresholds for physiological vs behavioral gains needs to be considered during combinatorial treatments.
Keywords: Acute intermittent hypoxia; Paired stimulation; Plasticity; Spinal cord injury.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures





References
-
- Arvanian VL, Mendell LM, 2001. Removal of NMDA receptor Mg(2+) block extends the action of NT-3 on synaptic transmission in neonatal rat motoneurons. J Neurophysiol 86, 123–129. - PubMed
-
- Bach KB, Mitchell GS, 1996. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respiration physiology 104, 251–260. - PubMed
-
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, 2004. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7, 48–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

