Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study
- PMID: 32987007
- PMCID: PMC7518804
- DOI: 10.1016/S0140-6736(20)32009-2
Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study
Abstract
Background: Many patients receiving dialysis in the USA share the socioeconomic characteristics of underserved communities, and undergo routine monthly laboratory testing, facilitating a practical, unbiased, and repeatable assessment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seroprevalence.
Methods: For this cross-sectional study, in partnership with a central laboratory that receives samples from approximately 1300 dialysis facilities across the USA, we tested the remainder plasma of 28 503 randomly selected adult patients receiving dialysis in July, 2020, using a spike protein receptor binding domain total antibody chemiluminescence assay (100% sensitivity, 99·8% specificity). We extracted data on age, sex, race and ethnicity, and residence and facility ZIP codes from the anonymised electronic health records, linking patient-level residence data with cumulative and daily cases and deaths per 100 000 population and with nasal swab test positivity rates. We standardised prevalence estimates according to the overall US dialysis and adult population, and present estimates for four prespecified strata (age, sex, region, and race and ethnicity).
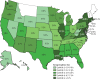
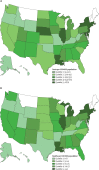
Findings: The sampled population had similar age, sex, and race and ethnicity distribution to the US dialysis population, with a higher proportion of older people, men, and people living in majority Black and Hispanic neighbourhoods than in the US adult population. Seroprevalence of SARS-CoV-2 was 8·0% (95% CI 7·7-8·4) in the sample, 8·3% (8·0-8·6) when standardised to the US dialysis population, and 9·3% (8·8-9·9) when standardised to the US adult population. When standardised to the US dialysis population, seroprevalence ranged from 3·5% (3·1-3·9) in the west to 27·2% (25·9-28·5) in the northeast. Comparing seroprevalent and case counts per 100 000 population, we found that 9·2% (8·7-9·8) of seropositive patients were diagnosed. When compared with other measures of SARS-CoV-2 spread, seroprevalence correlated best with deaths per 100 000 population (Spearman's ρ=0·77). Residents of non-Hispanic Black and Hispanic neighbourhoods experienced higher odds of seropositivity (odds ratio 3·9 [95% CI 3·4-4·6] and 2·3 [1·9-2·6], respectively) compared with residents of predominantly non-Hispanic white neighbourhoods. Residents of neighbourhoods in the highest population density quintile experienced increased odds of seropositivity (10·3 [8·7-12·2]) compared with residents of the lowest density quintile. County mobility restrictions that reduced workplace visits by at least 5% in early March, 2020, were associated with lower odds of seropositivity in July, 2020 (0·4 [0·3-0·5]) when compared with a reduction of less than 5%.
Interpretation: During the first wave of the COVID-19 pandemic, fewer than 10% of the US adult population formed antibodies against SARS-CoV-2, and fewer than 10% of those with antibodies were diagnosed. Public health efforts to limit SARS-CoV-2 spread need to especially target racial and ethnic minority and densely populated communities.
Funding: Ascend Clinical Laboratories.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
SARS-CoV-2 antibody seroprevalence in patients receiving dialysis in the USA.Lancet. 2020 Oct 24;396(10259):1308-1309. doi: 10.1016/S0140-6736(20)32006-7. Epub 2020 Sep 25. Lancet. 2020. PMID: 32987009 Free PMC article. No abstract available.
-
The seroprevalence of SARS-CoV-2 in patients on haemodialysis.Nat Rev Nephrol. 2021 Apr;17(4):225-226. doi: 10.1038/s41581-020-00379-y. Nat Rev Nephrol. 2021. PMID: 33230249 Free PMC article.
References
-
- Long QX, Liu BZ, Deng HJ. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. - PubMed
-
- US Food and Drug Administration EUA authorized serology test performance. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

