Role of POT1 in Human Cancer
- PMID: 32987645
- PMCID: PMC7598640
- DOI: 10.3390/cancers12102739
Role of POT1 in Human Cancer
Abstract
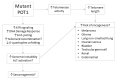
Telomere abnormalities facilitate cancer development by contributing to genomic instability and cellular immortalization. The Protection of Telomeres 1 (POT1) protein is an essential subunit of the shelterin telomere binding complex. It directly binds to single-stranded telomeric DNA, protecting chromosomal ends from an inappropriate DNA damage response, and plays a role in telomere length regulation. Alterations of POT1 have been detected in a range of cancers. Here, we review the biological functions of POT1, the prevalence of POT1 germline and somatic mutations across cancer predisposition syndromes and tumor types, and the dysregulation of POT1 expression in cancers. We propose a framework for understanding how POT1 abnormalities may contribute to oncogenesis in different cell types. Finally, we summarize the clinical implications of POT1 alterations in the germline and in cancer, and possible approaches for the development of targeted cancer therapies.
Keywords: POT1; alternative lengthening of telomeres; cancer; genomic instability; mutation; shelterin; telomerase; telomere; telomere length.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

