Mycotoxin Deoxynivalenol Has Different Impacts on Intestinal Barrier and Stem Cells by Its Route of Exposure
- PMID: 32987679
- PMCID: PMC7598581
- DOI: 10.3390/toxins12100610
Mycotoxin Deoxynivalenol Has Different Impacts on Intestinal Barrier and Stem Cells by Its Route of Exposure
Abstract
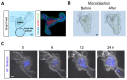
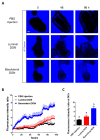
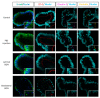
The different effects of deoxynivalenol (DON) on intestinal barrier and stem cells by its route of exposure remain less known. We explored the toxic effects of DON on intestinal barrier functions and stem cells after DON microinjection (luminal exposure) or addition to a culture medium (basolateral exposure) using three-dimensional mouse intestinal organoids (enteroids). The influx test using fluorescein-labeled dextran showed that basolateral DON exposure (1 micromolar (µM) disrupted intestinal barrier functions in enteroids compared with luminal DON exposure at the same concentration. Moreover, an immunofluorescence experiment of intestinal epithelial proteins, such as E-cadherin, claudin, zonula occludens-1 (ZO-1), and occludin, exhibited that only basolateral DON exposure broke down intestinal epithelial integrity. A time-lapse analysis using enteroids from leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5)-enhanced green fluorescence protein (EGFP) transgenic mice and 5-ethynyl-2-deoxyuridine (EdU) assay indicated that only the basolateral DON exposure, but not luminal DON exposure, suppressed Lgr5+ stem cell count and proliferative cell ratio, respectively. These results revealed that basolateral DON exposure has larger impacts on intestinal barrier function and stem cells than luminal DON exposure. This is the first report that DON had different impacts on intestinal stem cells depending on the administration route. In addition, RNA sequencing analysis showed different expression of genes among enteroids after basolateral and luminal DON exposure.
Keywords: basolateral exposure; deoxynivalenol; enteroids; intestinal barrier; intestinal stem cells; luminal exposure; microinjection; mycotoxin; organoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Luo X. Fusarium toxins contamination of cereals in China. JSM Mycotoxins. 1988;1988:97–98. doi: 10.2520/myco1975.1988.1Supplement_97. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

