Beyond the Canonical Endocannabinoid System. A Screening of PPAR Ligands as FAAH Inhibitors
- PMID: 32987725
- PMCID: PMC7582602
- DOI: 10.3390/ijms21197026
Beyond the Canonical Endocannabinoid System. A Screening of PPAR Ligands as FAAH Inhibitors
Abstract
In recent years, Peroxisome Proliferator-Activated Receptors (PPARs) have been connected to the endocannabinoid system. These nuclear receptors indeed mediate the effects of anandamide and similar substances such as oleoyl-ethanolamide and palmitoyl-ethanolamide. An increasing body of literature describing the interactions between the endocannabinoid system and PPARs has slowly but surely been accumulating over the past decade, and a multitarget approach involving these receptors and endocannabinoid degrading enzyme FAAH has been proposed for the treatment of inflammatory states, cancer, and Alzheimer's disease. The lack of knowledge about compounds endowed with such an activity profile therefore led us to investigate a library of readily available, well-characterized PPAR agonists that we had synthesized over the years in order to find a plausible lead compound for further development. Moreover, we propose a rationalization of our results via a docking study, which sheds some light on the binding mode of these PPAR agonists to FAAH and opens the way for further research in this field.
Keywords: Alzheimer’s disease; FAAH; PPARs; anandamide; endocannabinoids; inflammation; multitarget.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
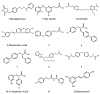

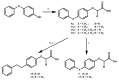
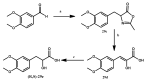
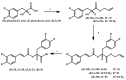
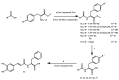
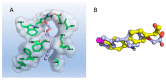
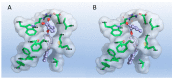

References
-
- Laghezza A., Montanari R., Lavecchia A., Piemontese L., Pochetti G., Iacobazzi V., Infantino V., Capelli D., De Bellis M., Liantonio A., et al. On the Metabolically Active Form of Metaglidasen: Improved Synthesis and Investigation of Its Peculiar Activity on Peroxisome Proliferator-Activated Receptors and Skeletal Muscles. ChemMedChem. 2015;10:555–565. doi: 10.1002/cmdc.201402462. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

