Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment
- PMID: 32987809
- PMCID: PMC7598644
- DOI: 10.3390/bios10100133
Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment
Abstract
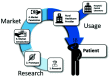
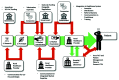
Point of care (PoC) diagnostics are at the focus of government initiatives, NGOs and fundamental research alike. In high-income countries, the hope is to streamline the diagnostic procedure, minimize costs and make healthcare processes more efficient and faster, which, in some cases, can be more a matter of convenience than necessity. However, in resource-limited settings such as low-income countries, PoC-diagnostics might be the only viable route, when the next laboratory is hours away. Therefore, it is especially important to focus research into novel diagnostics for these countries in order to alleviate suffering due to infectious disease. In this review, the current research describing the use of PoC diagnostics in resource-limited settings and the potential bottlenecks along the value chain that prevent their widespread application is summarized. To this end, we will look at literature that investigates different parts of the value chain, such as fundamental research and market economics, as well as actual use at healthcare providers. We aim to create an integrated picture of potential PoC barriers, from the first start of research at universities to patient treatment in the field. Results from the literature will be discussed with the aim to bring all important steps and aspects together in order to illustrate how effectively PoC is being used in low-income countries. In addition, we discuss what is needed to improve the situation further, in order to use this technology to its fullest advantage and avoid "leaks in the pipeline", when a promising device fails to take the next step of the valorization pathway and is abandoned.
Keywords: bottlenecks to usage by patients; low-income countries; point of care diagnostics; resource-limited settings.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Boutayeb A. The impact of infectious diseases on the development of Africa. In: Preedy V.R., Watson R.R., editors. The Handbook of Disease Burdens and Quality of Life Measures. Springer; New York, NY, USA: 2010. pp. 1171–1188.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

