A Selenophene-Incorporated Metal-Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity
- PMID: 32987864
- PMCID: PMC7582736
- DOI: 10.3390/molecules25194396
A Selenophene-Incorporated Metal-Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity
Abstract
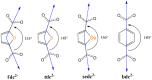
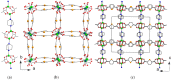
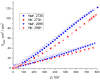
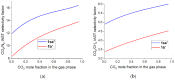
A new metal-organic coordination polymer [Zn2(sedc)2(dabco)] (1se; sedc2- = selepophene-2,5-dicarboxylate; dabco = 1,4-diazabicyclo[2.2.2]octane) was synthesized and characterized by single-crystal X-ray diffraction analysis. This MOF is based on {Zn2(OOCR)4N2} paddle wheels and is isoreticular to the family of [Zn2(bdc)2(dabco)] derivatives (1b; bdc2- = 1,4-benzenedicarboxylate) with pcu topology. The gas adsorption measurements revealed that 1se shows a 15% higher CO2 volumetric uptake at 273 K and 28% higher CO2 uptake at 298 K (both at 1 bar) compared to the prototypic framework 1b. Methane and nitrogen adsorption at 273 K was also investigated, and IAST calculations demonstrated a pronounced increase in CO2/CH4 and CO2/N2 selectivity for 1se, compared with 1b. For example, the selectivity factor for the equimolar CO2/CH4 gas mixture at 1 bar = 15.1 for 1se and 11.9 for 1b. The obtained results show a remarkable effect of the presence of selenium atom on the carbon dioxide affinity in the isoreticular metal-organic frameworks with very similar geometry and porosity.
Keywords: X-ray diffraction studies; adsorption measurements; gas adsorption selectivity; porous metal–organic frameworks; selenophene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang J., Huang L., Yang R., Zhang Z., Wu J., Gao Y., Wang Q., O’Hareb D., Zhong Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014;7:3478–3518. doi: 10.1039/C4EE01647E. - DOI
-
- Bhattacharyya D., Miller D.C. Post-combustion CO2 capture technologies—A review of processes for solvent-based and sorbent-based CO2 capture. Curr. Opin. Chem. Eng. 2017;17:78–92. doi: 10.1016/j.coche.2017.06.005. - DOI
-
- Leung D.Y.C., Caramanna G., Mercedes Maroto-Valer M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014;39:426–443. doi: 10.1016/j.rser.2014.07.093. - DOI
-
- Lin R.B., Li L., Alsalme A., Chen B. An Ultramicroporous Metal-Organic Framework for Sieving Separation of Carbon Dioxide from Methane. Small Struct. 2020 doi: 10.1002/sstr.202000022. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

