Comprehensive Lifestyle-Modification in Patients with Ulcerative Colitis-A Randomized Controlled Trial
- PMID: 32987894
- PMCID: PMC7599849
- DOI: 10.3390/jcm9103087
Comprehensive Lifestyle-Modification in Patients with Ulcerative Colitis-A Randomized Controlled Trial
Abstract
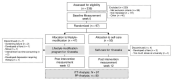
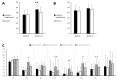
Patients with ulcerative colitis suffer from impaired health-related quality of life (HrQoL). Comprehensive lifestyle-modification might increase HrQoL and decrease disease activity. Ninety-seven patients in clinical remission with impaired HrQoL were randomly assigned to a 10 week comprehensive lifestyle-modification program (LSM; n = 47; 50.28 ± 11.90 years) or control (n = 50; 45.54 ± 12.49 years) that received a single workshop of intense training in naturopathic self-help strategies. Primary outcome was HrQoL (Inflammatory Bowel Disease Questionnaire; IBDQ) at week 12. Secondary outcomes included IBDQ subscales; generic HrQoL; disease activity and microbiome. Both groups showed improvement in HrQoL from baseline to post-treatment at week 12. The IBDQ sum score showed no significant group difference (p = 0.251). If patients attended more than 50% of the training sessions, a significant group effect (p = 0.034) was evident in favor of LSM. In addition, the SF-36 mental component summary (p = 0.002) was significantly different between the groups in favor of LSM. Disease activity microbiome and adverse events did not differ. Both a single workshop and a 10-week comprehensive lifestyle-modification program can improve HrQoL in patients with ulcerative colitis in remission with no apparent effects on clinical disease activity. A treatment difference was observed when examining a subsample of patients who attended ≥ 50% of sessions.
Keywords: health-related quality of life; integrative medicine; lifestyle-modification; randomized controlled trial; ulcerative colitis.
Conflict of interest statement
J.L. was a speaker for Repha GmbH, Techlab Inc., Falk Foundation, Takeda, Celegene GmbH and Willmar Schwabe and received research funding from Repha GmbH, Techlab Inc, Falk Foundation and Willmar Schwabe. No conflict of interest: Romy Lauche, Kerstin Kofink, Anna Katharina Koch, Anna Paul. The sponsors had no role in the design, execution, interpretation or writing of the study.
Figures



References
-
- Kucharzik T., Dignass A.U., Atreya R., Bokemeyer B., Esters P., Herrlinger K., Kannengießer K., Kienle P., Langhorst J., Lügering A., et al. Aktualisierte S3-Leitlinie Colitis ulcerosa der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) Z. für Gastroenterol. 2018;56:1087–1169. doi: 10.1055/a-0651-8174. - DOI - PubMed
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol I.E., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
-
- Langhorst J., Anthonisen I.B., Steder-Neukamm U., Lüdtke R., Spahn G., Michalsen A., Dobos G. Amount of systemic steroid medication is a strong predictor for the use of complementary and alternative medicine in patients with inflammatory bowel disease: Results from a German national survey. Inflamm. Bowel Dis. 2005;11:287–295. doi: 10.1097/01.MIB.0000160771.71328.6c. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

