Inhibitory Effects of a Reengineered Anthrax Toxin on Canine and Human Osteosarcoma Cells
- PMID: 32987941
- PMCID: PMC7601267
- DOI: 10.3390/toxins12100614
Inhibitory Effects of a Reengineered Anthrax Toxin on Canine and Human Osteosarcoma Cells
Abstract
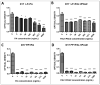
Canine and human osteosarcomas (OSA) share similarities. Novel therapies are necessary for these tumours. The Bacillus anthracis toxin was reengineered to target and kill cells with high expressions of matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPA). Since canine OSA express MMPs and uPA, we assessed whether the reengineered toxin could show efficacy against these tumours. Two OSA cell lines (canine D17 and human MG63) and a non-neoplastic canine osteoblastic cell line (COBS) were used. Cells were treated with different concentrations of the reengineered anthrax toxin and cell viability was quantified using MTT assay. The cell cycle, apoptosis, and necrosis were analysed by flow cytometry. The wound-healing assay was performed to quantify the migration capacity of treated cells. D17 and MG63 cells had significantly decreased viability after 24 h of treatment. Cell cycle analysis revealed that OSA cells underwent apoptosis when treated with the toxin, whereas COBS cells arrested in the G1 phase. The wound-healing assay showed that D17 and MG63 cells had a significantly reduced migration capacity after treatment. These results point for the first time towards the in vitro inhibitory effects of the reengineered anthrax toxin on OSA cells; this reengineered toxin could be further tested as a new therapy for OSA.
Keywords: Bacillus anthracis; anthrax; apoptosis; canine osteosarcoma; toxin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Withrow S.J., Powers B.E., Straw R.C., Wilkins R.M. Comparative aspects of osteosarcoma. Dog versus man. Clin. Orthop. Relat. Res. 1991:159–168. - PubMed
-
- Selvarajah G.T., Bonestroo F.A., Kirpensteijn J., Kik M.J., van der Zee R., van Eden W., Timmermans-Sprang E.P., Slob A., Mol J.A. Heat shock protein expression analysis in canine osteosarcoma reveals HSP60 as a potentially relevant therapeutic target. Cell Stress Chaperones. 2013;18:607–622. doi: 10.1007/s12192-013-0414-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

