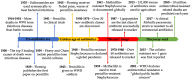
A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides
- PMID: 32987946
- PMCID: PMC7582481
- DOI: 10.3390/ijms21197047
A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides
Abstract
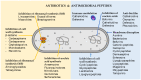
Antimicrobial resistance is a multifaceted crisis, imposing a serious threat to global health. The traditional antibiotic pipeline has been exhausted, prompting research into alternate antimicrobial strategies. Inspired by nature, antimicrobial peptides are rapidly gaining attention for their clinical potential as they present distinct advantages over traditional antibiotics. Antimicrobial peptides are found in all forms of life and demonstrate a pivotal role in the innate immune system. Many antimicrobial peptides are evolutionarily conserved, with limited propensity for resistance. Additionally, chemical modifications to the peptide backbone can be used to improve biological activity and stability and reduce toxicity. This review details the therapeutic potential of peptide-based antimicrobials, as well as the challenges needed to overcome in order for clinical translation. We explore the proposed mechanisms of activity, design of synthetic biomimics, and how this novel class of antimicrobial compound may address the need for effective antibiotics. Finally, we discuss commercially available peptide-based antimicrobials and antimicrobial peptides in clinical trials.
Keywords: antibiotic-resistance; antimicrobial activity; antimicrobial peptides; cationic peptides; clinical translation; peptide-based therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
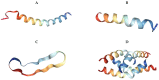
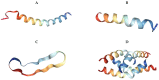
References
-
- O’Niel J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. HM Government and Welcome Trust; London, UK: 2016. Review on Antimicrobial Resistance.
-
- Hlth N.C.E., Stat N.C.H. Control of infectious diseases, 1900–1999. JAMA J. Am. Med. Assoc. 1999;282:1029–1032. reprinted in MMWR1999, 48, 621–629. - PubMed
-
- Fleming A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to Their Use in the Isolation of B. Influenzae. Br. J. Exp. Pathol. 1929;10:226–236. doi: 10.1093/clinids/2.1.129. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

