Recent Advances in the Synthesis and Application of Polymer Compartments for Catalysis
- PMID: 32987965
- PMCID: PMC7600123
- DOI: 10.3390/polym12102190
Recent Advances in the Synthesis and Application of Polymer Compartments for Catalysis
Abstract
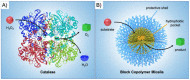
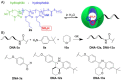
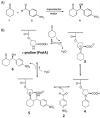
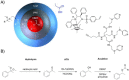
Catalysis is one of the most important processes in nature, science, and technology, that enables the energy efficient synthesis of essential organic compounds, pharmaceutically active substances, and molecular energy sources. In nature, catalytic reactions typically occur in aqueous environments involving multiple catalytic sites. To prevent the deactivation of catalysts in water or avoid unwanted cross-reactions, catalysts are often site-isolated in nanopockets or separately stored in compartments. These concepts have inspired the design of a range of synthetic nanoreactors that allow otherwise unfeasible catalytic reactions in aqueous environments. Since the field of nanoreactors is evolving rapidly, we here summarize-from a personal perspective-prominent and recent examples for polymer nanoreactors with emphasis on their synthesis and their ability to catalyze reactions in dispersion. Examples comprise the incorporation of catalytic sites into hydrophobic nanodomains of single chain polymer nanoparticles, molecular polymer nanoparticles, and block copolymer micelles and vesicles. We focus on catalytic reactions mediated by transition metal and organocatalysts, and the separate storage of multiple catalysts for one-pot cascade reactions. Efforts devoted to the field of nanoreactors are relevant for catalytic chemistry and nanotechnology, as well as the synthesis of pharmaceutical and natural compounds. Optimized nanoreactors will aid in the development of more potent catalytic systems for green and fast reaction sequences contributing to sustainable chemistry by reducing waste of solvents, reagents, and energy.
Keywords: block copolymers; cascade reactions; catalysis; controlled polymerization techniques; nanostructures; organocatalysis; polymer architectures; self-assembly; transition metal catalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












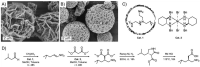

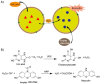

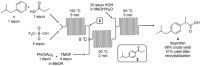
References
-
- Beller M., Renken A., van Santen R.A., editors. Catalysis: From Principles to Applications. Wiley-VCH; Weinheim, Germany: 2012.
-
- Lutz J.-F., Lehn J.-M., Meijer E.W., Matyjaszewski K. From precision polymers to complex materials and systems. Nat. Rev. Mater. 2016;1:16024. doi: 10.1038/natrevmats.2016.24. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

