Calcitonin gene related peptide monoclonal antibody treats headache in patients with active idiopathic intracranial hypertension
- PMID: 32988380
- PMCID: PMC7523364
- DOI: 10.1186/s10194-020-01182-7
Calcitonin gene related peptide monoclonal antibody treats headache in patients with active idiopathic intracranial hypertension
Abstract
Background: Headache is the dominant factor for quality of life related disability in idiopathic intracranial hypertension (IIH) and typically has migraine-like characteristics. There are currently no evidence-based therapeutics for headache in IIH, and consequently this is an important unmet clinical need.
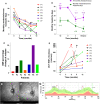
Case series: We report a series of seven patients in whom headaches were the presenting feature of IIH and the headaches had migraine-like characteristics, as is typical in many IIH patients. Papilloedema settled (ocular remission) but headaches continued. These headaches responded markedly to erenumab, a monoclonal antibody targeted against the calcitonin gene related peptide (CGRP) receptor. Of note, there was a recurrence of raised ICP, as evidenced by a return of the papilloedema, however the headaches did not recur whilst treated with erenumab.
Conclusions: Those with prior IIH who have their headaches successfully treated with CGRP therapy, should remain under close ocular surveillance (particularly when weight gain is evident) as papilloedema can re-occur in the absence of headache. These cases may suggest that CGRP could be a mechanistic driver for headache in patients with active IIH.
Keywords: CGRP monoclonal antibody; Headache; Idiopathic intracranial hypertension; Papilloedema; Raised intracranial pressure.
Conflict of interest statement
Edwards has received speaker fees and Honoria from Novartis, Teva, Eli Lily and Allergan on headache treatments but not related to IIH. Mollan has received Honoria from Novartis for speaking on topics unrelated to this drug, but within a National headache network meeting (November 2019). Sinclair has received speaker fees and Honoraria from Novartis (erenumab) and Allergan (BOTOX), in addition, Invex therapeutics, company director with salary and stock options (2019, 2020). Grech, Consultancy work for Invex therapeutics (2020). Authors declare no other financial relationships with any organisations that might have an interest in the submitted work; and no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, Krishnan A, Chavda SV, Ramalingam S, Edwards J, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89(10):1088–1100. doi: 10.1136/jnnp-2017-317440. - DOI - PMC - PubMed
-
- Mollan S, Hemmings K, Herd CP, Denton A, Williamson S, Sinclair AJ. What are the research priorities for idiopathic intracranial hypertension? A priority setting partnership between patients and healthcare professionals. BMJ Open. 2019;9(3):e026573. doi: 10.1136/bmjopen-2018-026573. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

