Inhibition of αvβ3 integrin impairs adhesion and uptake of tumor-derived small extracellular vesicles
- PMID: 32988382
- PMCID: PMC7520983
- DOI: 10.1186/s12964-020-00630-w
Inhibition of αvβ3 integrin impairs adhesion and uptake of tumor-derived small extracellular vesicles
Abstract
Background: Extracellular vesicles (EVs) are lipid-bound particles that are naturally released from cells and mediate cell-cell communication. Integrin adhesion receptors are enriched in small EVs (SEVs) and SEV-carried integrins have been shown to promote cancer cell migration and to mediate organ-specific metastasis; however, how integrins mediate these effects is not entirely clear and could represent a combination of EV binding to extracellular matrix and cells.
Methods: To probe integrin role in EVs binding and uptake, we employed a disintegrin inhibitor (DisBa-01) of integrin binding with specificity for αvβ3 integrin. EVs were purified from MDA-MB-231 cells conditioned media by serial centrifugation method. Isolated EVs were characterized by different techniques and further employed in adhesion, uptake and co-culture experiments.
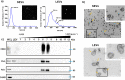
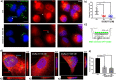
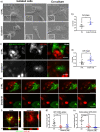
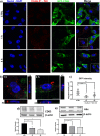
Results: We find that SEVs secreted from MDA-MB-231 breast cancer cells carry αvβ3 integrin and bind directly to fibronectin-coated plates, which is inhibited by DisBa-01. SEV coating on tissue culture plates also induces adhesion of MDA-MB-231 cells, which is inhibited by DisBa-01 treatment. Analysis of EV uptake and interchange between cells reveals that the amount of CD63-positive EVs delivered from malignant MDA-MB-231 breast cells to non-malignant MCF10A breast epithelial cells is reduced by DisBa-01 treatment. Inhibition of αvβ3 integrin decreases CD63 expression in cancer cells suggesting an effect on SEV content.
Conclusion: In summary, our findings demonstrate for the first time a key role of αvβ3 integrin in cell-cell communication through SEVs. Video Abstract.
Keywords: Adhesion; Breast cancer; Small extracellular vesicles; Uptake; αvβ3 integrin.
Conflict of interest statement
The authors declare that they have no competing interests. The work was funded in part by National Institute of Health [NIH grants: 1R01GM117916 and 1R01CA206458 to AMW].
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

