Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology
- PMID: 32988409
- PMCID: PMC7523295
- DOI: 10.1186/s13195-020-00682-7
Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology
Abstract
Background: Blood-based biomarkers for Alzheimer's disease (AD) might facilitate identification of participants for clinical trials targeting amyloid beta (Abeta) accumulation, and aid in AD diagnostics. We examined the potential of plasma markers Abeta(1-42/1-40), glial fibrillary acidic protein (GFAP) and neurofilament light (NfL) to identify cerebral amyloidosis and/or disease severity.
Methods: We included individuals with a positive (n = 176: 63 ± 7 years, 87 (49%) females) or negative (n = 76: 61 ± 9 years, 27 (36%) females) amyloid PET status, with syndrome diagnosis subjective cognitive decline (18 PET+, 25 PET-), mild cognitive impairment (26 PET+, 24 PET-), or AD-dementia (132 PET+). Plasma Abeta(1-42/1-40), GFAP, and NfL were measured by Simoa. We applied two-way ANOVA adjusted for age and sex to investigate the associations of the plasma markers with amyloid PET status and syndrome diagnosis; logistic regression analysis with Wald's backward selection to identify an optimal panel that identifies amyloid PET positivity; age, sex, and education-adjusted linear regression analysis to investigate associations between the plasma markers and neuropsychological test performance; and Spearman's correlation analysis to investigate associations between the plasma markers and medial temporal lobe atrophy (MTA).
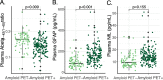
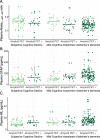
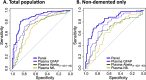
Results: Abeta(1-42/1-40) and GFAP independently associated with amyloid PET status (p = 0.009 and p < 0.001 respectively), and GFAP and NfL independently associated with syndrome diagnosis (p = 0.001 and p = 0.048 respectively). The optimal panel identifying a positive amyloid status included Abeta(1-42/1-40) and GFAP, alongside age and APOE (AUC = 88% (95% CI 83-93%), 82% sensitivity, 86% specificity), while excluding NfL and sex. GFAP and NfL robustly associated with cognitive performance on global cognition and all major cognitive domains (GFAP: range standardized β (sβ) = - 0.40 to - 0.26; NfL: range sβ = - 0.35 to - 0.18; all: p < 0.002), whereas Abeta(1-42/1-40) associated with global cognition, memory, attention, and executive functioning (range sβ = 0.22 - 0.11; all: p < 0.05) but not language. GFAP and NfL showed moderate positive correlations with MTA (both: Spearman's rho> 0.33, p < 0.001). Abeta(1-42/1-40) showed a moderate negative correlation with MTA (Spearman's rho = - 0.24, p = 0.001).
Discussion and conclusions: Combination of plasma Abeta(1-42/1-40) and GFAP provides a valuable tool for the identification of amyloid PET status. Furthermore, plasma GFAP and NfL associate with various disease severity measures suggesting potential for disease monitoring.
Keywords: Alzheimer’s continuum; Amyloid pathology; Blood-based biomarkers; Plasma GFAP; Plasma amyloid beta.
Conflict of interest statement
IV, ET, JK, AdW, MZ, SV, RO, BvB, and WvdF report no financial disclosures or conflicts of interest. KM, JV, and ES are employees of ADx Neurosciences NV and report no financial disclosures or conflicts of interest. HV is a co-founder of ADx NeuroSciences NV and a founder of Biomarkable bvba. FB has received consultancy fees from Roche, Biogen, Merck, IXICO, Bayer, and Novartis. PS has received consultancy/speaker fees (paid to the institution) from Biogen, People Bio, Roche (Diagnostics), Novartis Cardiology. He is PI of studies with Probiodrug, EIP Pharma, IONIS, CogRx, AC Immune, and Toyama. CT has a collaboration contract with ADx Neurosciences, performed contract research or received grants from Probiodrug, AC Immune, Biogen-Esai, CogRx, Toyama, Janssen prevention center, Boehringer, AxonNeurosciences, Fujirebio, EIP farma, PeopleBio, Roche.
Figures


References
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

