Epidemiology of age-dependent prevalence of Bovine Herpes Virus Type 1 (BoHV-1) in dairy herds with and without vaccination
- PMID: 32988417
- PMCID: PMC7520977
- DOI: 10.1186/s13567-020-00842-5
Epidemiology of age-dependent prevalence of Bovine Herpes Virus Type 1 (BoHV-1) in dairy herds with and without vaccination
Abstract
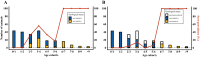
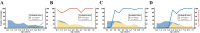
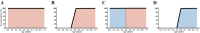
Many studies report age as a risk factor for BoHV-1 infection or seropositivity. However, it is unclear whether this pattern reflects true epidemiological causation or is a consequence of study design and other issues. Here, we seek to understand the age-related dynamics of BoHV-1 seroprevalence in seasonal calving Irish dairy herds and provide decision support for the design and implementation of effective BoHV-1 testing strategies. We analysed seroprevalence data from dairy herds taken during two Irish seroprevalence surveys conducted between 2010 and 2017. Age-dependent seroprevalence profiles were constructed for herds that were seropositive and unvaccinated. Some of these profiles revealed a sudden increase in seroprevalence between adjacent age-cohorts, from absent or low to close to 100% of seropositive animals. By coupling the outcome of our data analysis with simulation output of an individual-based model at the herd scale, we have shown that these sudden increases are related to extensive virus circulation within a herd for a limited time, which may then subsequently remain latent over the following years. BoHV-1 outbreaks in dairy cattle herds affect animals independent of age and lead to almost 100% seroconversion in all age groups, or at least in all animals within a single epidemiological unit. In the absence of circulating infection, there is a year-on-year increase in the age-cohort at which seroprevalence changes from low to high. The findings of this study inform recommendations regarding testing regimes in the context of contingency planning or an eradication programme in seasonal calving dairy herds.
Keywords: Ireland; dairy; infection dynamics; infectious Bovine Rhinotracheitis (IBR); seasonal calving; serosurveillance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Nettleton P, Russell G. Update on infectious bovine rhinotracheitis. Pract. 2017;39:255–272. doi: 10.1136/inp.j2226. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

