The Anticancer Potential of T Cell Receptor-Engineered T Cells
- PMID: 32988787
- PMCID: PMC7770096
- DOI: 10.1016/j.trecan.2020.09.002
The Anticancer Potential of T Cell Receptor-Engineered T Cells
Abstract
Adoptively transferred T cell receptor (TCR)-transgenic T cells (TCR-T cells) are not restricted by cell surface expression of their targets and are therefore poised to become a main pillar of cellular cancer immunotherapies. Addressing clinical and laboratory data, we discuss emerging features for the efficient deployment of novel TCR-T therapies, such as selection of ideal TCRs targeting validated epitopes with well-characterized cancer cell expression and processing, enhancing TCR-T effector function, trafficking, expansion, persistence, and memory formation by strategic selection of substrate cells, and gene-engineering with synthetic co-stimulatory circuits. Overall, a better understanding of the relevant mechanisms of action and resistance will help prioritize the vast array of potential TCR-T optimizations for future clinical products.
Keywords: TCR-T; cancer immunotherapy; engineered T cells.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
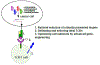
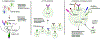
References
-
- Wei SC et al. (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

