First-in-Human Trial of the Oral Ataxia Telangiectasia and RAD3-Related (ATR) Inhibitor BAY 1895344 in Patients with Advanced Solid Tumors
- PMID: 32988960
- PMCID: PMC9554790
- DOI: 10.1158/2159-8290.CD-20-0868
First-in-Human Trial of the Oral Ataxia Telangiectasia and RAD3-Related (ATR) Inhibitor BAY 1895344 in Patients with Advanced Solid Tumors
Abstract
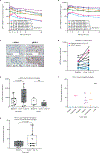
Targeting the ataxia telangiectasia and RAD3-related (ATR) enzyme represents a promising anticancer strategy for tumors with DNA damage response (DDR) defects and replication stress, including inactivation of ataxia telangiectasia mutated (ATM) signaling. We report the dose-escalation portion of the phase I first-in-human trial of oral ATR inhibitor BAY 1895344 intermittently dosed 5 to 80 mg twice daily in 21 patients with advanced solid tumors. The MTD was 40 mg twice daily 3 days on/4 days off. Most common adverse events were manageable and reversible hematologic toxicities. Partial responses were achieved in 4 patients and stable disease in 8 patients. Median duration of response was 315.5 days. Responders had ATM protein loss and/or deleterious ATM mutations and received doses ≥40 mg twice daily. Overall, BAY 1895344 is well tolerated, with antitumor activity against cancers with certain DDR defects, including ATM loss. An expansion phase continues in patients with DDR deficiency. SIGNIFICANCE: Oral BAY 1895344 was tolerable, with antitumor activity in heavily pretreated patients with various advanced solid tumors, particularly those with ATM deleterious mutations and/or loss of ATM protein; pharmacodynamic results supported a mechanism of action of increased DNA damage. Further study is warranted in this patient population.See related commentary by Italiano, p. 14.This article is highlighted in the In This Issue feature, p. 1.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures

Comment in
-
ATR Inhibition as an Attractive Therapeutic Resource against Cancer.Cancer Discov. 2021 Jan;11(1):14-16. doi: 10.1158/2159-8290.CD-20-1354. Cancer Discov. 2021. PMID: 34003779
Comment on
-
ATR Inhibition as an Attractive Therapeutic Resource against Cancer.Cancer Discov. 2021 Jan;11(1):14-16. doi: 10.1158/2159-8290.CD-20-1354. Cancer Discov. 2021. PMID: 34003779
References
-
- Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell 2017;66:801–17. - PubMed
-
- Weber AM, Ryan AJ. ATM and ATR as therapeutic targets in cancer. Pharmacol Ther 2015;149:124–38. - PubMed
-
- Durocher D, Jackson SP. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol 2001;13:225–31. - PubMed
-
- de Klein A, Muijtjens M, van Os R, Verhoeven Y, Smit B, Carr AM, et al. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol 2000;10:479–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

