Influenza Immunization in the Context of Preexisting Immunity
- PMID: 32988981
- PMCID: PMC8559541
- DOI: 10.1101/cshperspect.a040964
Influenza Immunization in the Context of Preexisting Immunity
Abstract
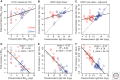
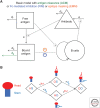
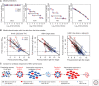
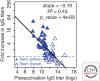
Although we develop influenza immunity from an early age, it is insufficient to prevent future infection with antigenically novel strains. One proposed way to generate long-term protective immunity against a broad range of influenza virus strains is to boost responses to the conserved epitopes on the hemagglutinin, the major surface glycoprotein on the influenza virus. Influenza-specific humoral immunity comprises a large fraction of the overall immune memory in humans, and it has been long recognized that preexisting immunity to influenza shapes the response to subsequent influenza infections and vaccinations. However, the mechanisms by which preexisting immunity modulates the response to influenza vaccination are still not completely understood. Using a set of mathematical models, we explore several hypotheses that may contribute to diminished boosting of antibodies to conserved epitopes after repeated vaccinations.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Bodewes R, de Mutsert G, van der Klis FR, Ventresca M, Wilks S, Smith DJ, Koopmans M, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2011. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin Vaccine Immunol 18: 469–476. 10.1128/CVI.00396-10 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention (CDC). 2009. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep 58: 521–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
