Upregulation of nAChRs and Changes in Excitability on VTA Dopamine and GABA Neurons Correlates to Changes in Nicotine-Reward-Related Behavior
- PMID: 32988984
- PMCID: PMC7568605
- DOI: 10.1523/ENEURO.0189-20.2020
Upregulation of nAChRs and Changes in Excitability on VTA Dopamine and GABA Neurons Correlates to Changes in Nicotine-Reward-Related Behavior
Abstract
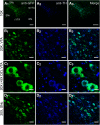
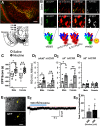
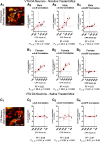
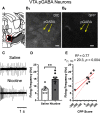
Previous reports indicate that nicotine reward is mediated through α4β2*, α6β2*, and α4α6β2* nicotinic acetylcholine receptors (nAChRs; * indicates that additional nAChR subunits may be present). Little is known about α4α6β2* nAChR involvement in reward and reinforcement because of a lack of methods that allow the direct investigation of this particular nAChR subtype. Here, we use male and female mice that contain α4-mCherry and α6-GFP nAChR subunits to show that concentrations of nicotine sufficient to evoke reward-related behavior robustly upregulate α4* and α4α6* nAChRs on midbrain dopamine (DA) and GABA neurons. Furthermore, the extent of α4α6* nAChR upregulation on ventral tegmental area (VTA) DA neurons aligns with the magnitude of nicotine reward-related behavior. We also show that the upregulation of nAChRs is accompanied by a functional change in firing frequency of both DA and GABA neurons in the VTA that is directly linked to nicotine reward-related behavior.
Keywords: excitability; nicotine; nicotinic receptor; reward; upregulation.
Copyright © 2020 Akers et al.
Figures


References
-
- Avelar AJ, Akers AT, Baumgard ZJ, Cooper SY, Casinelli GP, Henderson BJ (2019) Why flavored vape products may be attractive: green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function. Neuropharmacology 158:107729 10.1016/j.neuropharm.2019.107729 - DOI - PMC - PubMed
-
- Engle SE, Shih PY, McIntosh JM, Drenan RM (2013) α4α6β2* nicotinic acetylcholine receptor activation on ventral tegmental area dopamine neurons is sufficient to stimulate a depolarizing conductance and enhance surface AMPA receptor function. Mol Pharmacol 84:393–406. 10.1124/mol.113.087346 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
