Alternative conformations and motions adopted by 30S ribosomal subunits visualized by cryo-electron microscopy
- PMID: 32989043
- PMCID: PMC7668263
- DOI: 10.1261/rna.075846.120
Alternative conformations and motions adopted by 30S ribosomal subunits visualized by cryo-electron microscopy
Abstract
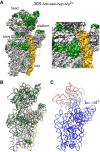
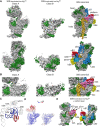
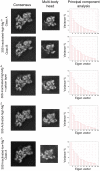
It is only after recent advances in cryo-electron microscopy that it is now possible to describe at high-resolution structures of large macromolecules that do not crystalize. Purified 30S subunits interconvert between an "active" and "inactive" conformation. The active conformation was described by crystallography in the early 2000s, but the structure of the inactive form at high resolution remains unsolved. Here we used cryo-electron microscopy to obtain the structure of the inactive conformation of the 30S subunit to 3.6 Å resolution and study its motions. In the inactive conformation, an alternative base-pairing of three nucleotides causes the region of helix 44, forming the decoding center to adopt an unlatched conformation and the 3' end of the 16S rRNA positions similarly to the mRNA during translation. Incubation of inactive 30S subunits at 42°C reverts these structural changes. The air-water interface to which ribosome subunits are exposed during sample preparation also peel off some ribosomal proteins. Extended exposures to low magnesium concentrations make the ribosomal particles more susceptible to the air-water interface causing the unfolding of large rRNA structural domains. Overall, this study provides new insights about the conformational space explored by the 30S ribosomal subunit when the ribosomal particles are free in solution.
Keywords: 30S subunit; cryo-electron microscopy; ribosome structure.
© 2020 Jahagirdar et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures



References
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
