Influence of Shigella flexneri 2a O Antigen Acetylation on Its Bacteriophage Sf6 Receptor Activity and Bacterial Interaction with Human Cells
- PMID: 32989087
- PMCID: PMC7685545
- DOI: 10.1128/JB.00363-20
Influence of Shigella flexneri 2a O Antigen Acetylation on Its Bacteriophage Sf6 Receptor Activity and Bacterial Interaction with Human Cells
Abstract
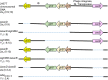
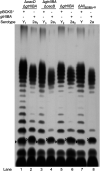
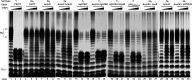
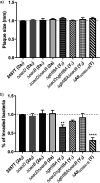
Shigella flexneri is a major causative agent of bacillary dysentery in developing countries, where serotype 2a2 is the prevalent strain. To date, approximately 30 serotypes have been identified for S. flexneri, and the major contribution to the emergence of new serotypes is chemical modifications of the lipopolysaccharide (LPS) component O antigen (Oag). Glucosylation, O-acetylation, and phosphoethanolamine (PEtN) modifications increase the Oag diversity, providing benefits to S. flexneri LPS Oag acts as a primary receptor for bacteriophage Sf6, which infects only a limited range of S. flexneri serotypes (Y and X). It uses its tailspike protein (Sf6TSP) to establish initial interaction with LPS Oags that it then hydrolyzes. Currently, there is a lack of comprehensive study on the parent and serotype variant strains from the same genetic background and an understanding of the importance of LPS Oag O-acetylations. Therefore, a set of isogenic strains (based on S. flexneri 2457T [2a2]) with deletions of different Oag modification genes (oacB, oacD, and gtrII) that resemble different naturally occurring serotype Y and 2a strains was created. The impacts of these Oag modifications on S. flexneri sensitivity to Sf6 and the pathogenesis-related properties were then compared. We found that Sf6TSP can hydrolyze serotype 2a LPS Oag, identified that 3/4-O-acetylation is essential for resistance of serotype 2a strains to Sf6, and showed that serotype 2a strains have better invasion ability. Lastly, we revealed two new serotype conversions for S. flexneri, thereby contributing to understanding the evolution of this important human pathogen.IMPORTANCE The emergence of antibiotic-resistant strains and lack of efficient vaccines have made Shigella a priority organism for the World Health Organization (1). Therefore, bacteriophage therapy has received increasing attention as an alternative therapeutic approach. LPS Oag is the most variable part of LPS due to chemical modifications and is the target of bacteriophage Sf6 (S. flexneri specific). We dissected the evolution of S. flexneri serotype Y to 2a2, which revealed a new role for a gene acquired during serotype conversion and furthermore identified new specific forms of LPS receptor for Sf6. Collectively, these results unfold the importance of the acquisition of those Oag modification genes and further our understanding of the relationship between Sf6 and S. flexneri.
Keywords: O antigen; O-acetylation; Sf6; Shigella flexneri; bacteriophages; glucosylation; lipopolysaccharide; serotypes.
Copyright © 2020 American Society for Microbiology.
Figures




References
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi:10.1016/S1473-3099(17)30753-3. - DOI - PubMed
-
- Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, Kotloff KL, Levine MM, Luby SP, MacLennan CA, Pan WK, Pavlinac PB, Platts-Mills JA, Qadri F, Riddle MS, Ryan ET, Shoultz DA, Steele AD, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC Jr. 2018. Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis 18:1229–1240. doi:10.1016/S1473-3099(18)30475-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

