Prefrontal Neural Ensembles Develop Selective Code for Stimulus Associations within Minutes of Novel Experiences
- PMID: 32989098
- PMCID: PMC7577596
- DOI: 10.1523/JNEUROSCI.1503-20.2020
Prefrontal Neural Ensembles Develop Selective Code for Stimulus Associations within Minutes of Novel Experiences
Abstract
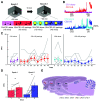
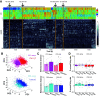
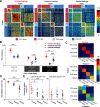
Prevailing theories posit that the hippocampus rapidly learns stimulus conjunctions during novel experiences, whereas the neocortex learns slowly through subsequent, off-line interaction with the hippocampus. Parallel evidence, however, shows that the medial prefrontal cortex (mPFC; a critical node of the neocortical network supporting long-term memory storage) undergoes rapid modifications of gene expression, synaptic structure, and physiology at the time of encoding. These observations, along with impaired learning with disrupted mPFC, suggest that mPFC neurons may exhibit rapid neural plasticity during novel experiences; however, direct empirical evidence is lacking. We extracellularly recorded action potentials of cells in the prelimbic region of the mPFC, while male rats received a sequence of stimulus presentations for the first time in life. Moment-to-moment tracking of neural ensemble firing patterns revealed that the prelimbic network activity exhibited an abrupt transition within 1 min after the first encounter of an aversive but not neutral stimulus. This network-level change was driven by ∼15% of neurons that immediately elevated their spontaneous firing rates (FRs) and developed firing responses to a neutral stimulus preceding the aversive stimulus within a few instances of their pairings. When a new sensory stimulus was paired with the same aversive stimulus, about half of these neurons generalized firing responses to the new stimulus association. Thus, prelimbic neurons are capable of rapidly forming ensemble codes for novel stimulus associations within minutes. This circuit property may enable the mPFC to rapidly detect and selectively encode the central content of novel experiences.SIGNIFICANCE STATEMENT During a new experience, a region of the brain, called the hippocampus, rapidly forms its memory and later instructs another region, called the neocortex, that stores its content. Consistent with this dominant view, cells in the neocortex gradually strengthen the selectivity for the memory content over weeks after novel experiences. However, we still do not know precisely when these cells begin to develop the selectivity. We found that neocortical cells were capable of forming the selectivity for ongoing events within a few minutes of new experiences. This finding provides support for an alternative view that the neocortex works with, but not follows, the hippocampus to form new memories.
Keywords: consolidation; encoding; hippocampus; plasticity; prefrontal cortex; rats.
Copyright © 2020 the authors.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
