The dependency of red Rubisco on its cognate activase for enhancing plant photosynthesis and growth
- PMID: 32989135
- PMCID: PMC7568259
- DOI: 10.1073/pnas.2011641117
The dependency of red Rubisco on its cognate activase for enhancing plant photosynthesis and growth
Abstract
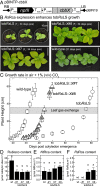
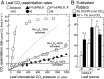
Plant photosynthesis and growth are often limited by the activity of the CO2-fixing enzyme Rubisco. The broad kinetic diversity of Rubisco in nature is accompanied by differences in the composition and compatibility of the ancillary proteins needed for its folding, assembly, and metabolic regulation. Variations in the protein folding needs of catalytically efficient red algae Rubisco prevent their production in plants. Here, we show this impediment does not extend to Rubisco from Rhodobacter sphaeroides (RsRubisco)-a red-type Rubisco able to assemble in plant chloroplasts. In transplastomic tobRsLS lines expressing a codon optimized Rs-rbcLS operon, the messenger RNA (mRNA) abundance was ∼25% of rbcL transcript and RsRubisco ∼40% the Rubisco content in WT tobacco. To mitigate the low activation status of RsRubisco in tobRsLS (∼23% sites active under ambient CO2), the metabolic repair protein RsRca (Rs-activase) was introduced via nuclear transformation. RsRca production in the tobRsLS::X progeny matched endogenous tobacco Rca levels (∼1 µmol protomer·m2) and enhanced RsRubisco activation to 75% under elevated CO2 (1%, vol/vol) growth. Accordingly, the rate of photosynthesis and growth in the tobRsLS::X lines were improved >twofold relative to tobRsLS. Other tobacco lines producing RsRubisco containing alternate diatom and red algae S-subunits were nonviable as CO2-fixation rates (kcatc) were reduced >95% and CO2/O2 specificity impaired 30-50%. We show differences in hybrid and WT RsRubisco biogenesis in tobacco correlated with assembly in Escherichia coli advocating use of this bacterium to preevaluate the kinetic and chloroplast compatibility of engineered RsRubisco, an isoform amenable to directed evolution.
Keywords: Rubisco activase; carbon fixation; chloroplast transformation; photosynthesis.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Grafting Rhodobacter sphaeroides with red algae Rubisco to accelerate catalysis and plant growth.Nat Plants. 2023 Jun;9(6):978-986. doi: 10.1038/s41477-023-01436-7. Epub 2023 Jun 8. Nat Plants. 2023. PMID: 37291398
-
Directed Evolution of an Improved Rubisco; In Vitro Analyses to Decipher Fact from Fiction.Int J Mol Sci. 2019 Oct 10;20(20):5019. doi: 10.3390/ijms20205019. Int J Mol Sci. 2019. PMID: 31658746 Free PMC article.
-
An improved Escherichia coli screen for Rubisco identifies a protein-protein interface that can enhance CO2-fixation kinetics.J Biol Chem. 2018 Jan 5;293(1):18-27. doi: 10.1074/jbc.M117.810861. Epub 2017 Oct 6. J Biol Chem. 2018. PMID: 28986448 Free PMC article.
-
Improving photosynthesis through the enhancement of Rubisco carboxylation capacity.Biochem Soc Trans. 2021 Nov 1;49(5):2007-2019. doi: 10.1042/BST20201056. Biochem Soc Trans. 2021. PMID: 34623388 Review.
-
Biogenesis and Metabolic Maintenance of Rubisco.Annu Rev Plant Biol. 2017 Apr 28;68:29-60. doi: 10.1146/annurev-arplant-043015-111633. Epub 2017 Jan 11. Annu Rev Plant Biol. 2017. PMID: 28125284 Review.
Cited by
-
Predicting plant Rubisco kinetics from RbcL sequence data using machine learning.J Exp Bot. 2023 Jan 11;74(2):638-650. doi: 10.1093/jxb/erac368. J Exp Bot. 2023. PMID: 36094849 Free PMC article.
-
Recent advancements in CRISPR/Cas technology for accelerated crop improvement.Planta. 2022 Apr 23;255(5):109. doi: 10.1007/s00425-022-03894-3. Planta. 2022. PMID: 35460444 Review.
-
Knowledge of microalgal Rubiscos helps to improve photosynthetic efficiency of crops.Planta. 2025 Mar 5;261(4):78. doi: 10.1007/s00425-025-04645-w. Planta. 2025. PMID: 40042639 Review.
-
Grafting Rhodobacter sphaeroides with red algae Rubisco to accelerate catalysis and plant growth.Nat Plants. 2023 Jun;9(6):978-986. doi: 10.1038/s41477-023-01436-7. Epub 2023 Jun 8. Nat Plants. 2023. PMID: 37291398
-
Evolution-aided engineering of plant specialized metabolism.aBIOTECH. 2021 Jun 19;2(3):240-263. doi: 10.1007/s42994-021-00052-3. eCollection 2021 Sep. aBIOTECH. 2021. PMID: 36303885 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

