Strength of tonic T cell receptor signaling instructs T follicular helper cell-fate decisions
- PMID: 32989327
- PMCID: PMC7578106
- DOI: 10.1038/s41590-020-0781-7
Strength of tonic T cell receptor signaling instructs T follicular helper cell-fate decisions
Abstract
T follicular helper (TFH) cells are critical in adaptive immune responses to pathogens and vaccines; however, what drives the initiation of their developmental program remains unclear. Studies suggest that a T cell antigen receptor (TCR)-dependent mechanism may be responsible for the earliest TFH cell-fate decision, but a critical aspect of the TCR has been overlooked: tonic TCR signaling. We hypothesized that tonic signaling influences early TFH cell development. Here, two murine TCR-transgenic CD4+ T cells, LLO56 and LLO118, which recognize the same antigenic peptide presented on major histocompatibility complex molecules but experience disparate strengths of tonic signaling, revealed low tonic signaling promotes TFH cell differentiation. Polyclonal T cells paralleled these findings, with naive Nur77 expression distinguishing TFH cell potential. Two mouse lines were also generated to both increase and decrease tonic signaling strength, directly establishing an inverse relationship between tonic signaling strength and TFH cell development. Our findings elucidate a central role for tonic TCR signaling in early TFH cell-lineage decisions.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures

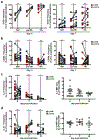
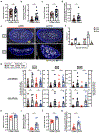


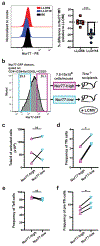
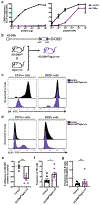
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

