Agaricus blazei extract (FA-2-b-β) induces apoptosis in chronic myeloid leukemia cells
- PMID: 32989404
- PMCID: PMC7517625
- DOI: 10.3892/ol.2020.12133
Agaricus blazei extract (FA-2-b-β) induces apoptosis in chronic myeloid leukemia cells
Abstract
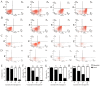
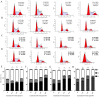
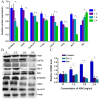
Agaricus blazei Murill (AbM) is a mushroom belonging to the Basidiomycetes family, which is believed to have antitumor and antioxidative activities. Proteoglycans and ergosterol are considered the key compounds of AbM for antitumor properties and so are used in complementary and alternative medicine as an anticancer drug. AbM is used to avoid serious side effects that would inevitably affect patients. Currently, the efficacy of AbM against chronic myeloid leukemia (CML) has not been established. The present study aimed to investigate the antitumor activities of the acidic RNA protein complex, FA-2-b-β, extracted from wild edible AbM. The CML K562 cells or primary CML bone marrow (BM) cells were treated with FA-2-b-β at different concentrations and time points. CML cell line proliferation and apoptosis were determined using the CCK-8 assay or Annexin V/propidium iodide (PI) labeling, RT-qPCR and western blotting was performed to determine the involvement of the Wnt/β-catenin-associated apoptotic pathway. The results of the present study demonstrated that FA-2-b-β has a high anti-proliferative potency and strong pro-apoptotic effects. Thus, daily intake of mushrooms containing FA-2-b-β may be an adequate source as an alternative medicine in the management of CML, and may provide useful information for the development of a novel therapeutic target in this area.
Keywords: AbM; CML; Wnt/β-catenin signaling pathway; apoptosis cycle.
Copyright: © Sun et al.
Figures


References
-
- Zhou H, Mak PY, Mu H, Mak DH, Zeng Z, Cortes J, Liu Q, Andreeff M, Carter BZ. Combined inhibition of β-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia. 2017;31:2065–2074. doi: 10.1038/leu.2017.87. - DOI - PMC - PubMed
-
- Xiao X, Liu P, Li D, Xia Z, Wang P, Zhang X, Liu M, Liao L, Jiao B, Ren R. Combination therapy of BCR-ABL-positive B cell acute lymphoblastic leukemia by tyrosine kinase inhibitor dasatinib and c-JUN N-terminal kinase inhibition. J Hematol Oncol. 2020;13:80. doi: 10.1186/s13045-020-00912-3. - DOI - PMC - PubMed
-
- Zovko A, Yektaei-Karin E, Daniel S, Nilsson A, Wallvik J, Stenke L. Montelukast, a cysteinyl leukotriene receptor antagonist, inhibits the growth of chronic myeloid leukemia cells through apoptosis. Oncol Rep. 2018;40:902–908. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
