A Systematic Review and Mixed Treatment Comparison of Pharmaceutical Interventions for Multiple Sclerosis
- PMID: 32989721
- PMCID: PMC7606402
- DOI: 10.1007/s40120-020-00212-5
A Systematic Review and Mixed Treatment Comparison of Pharmaceutical Interventions for Multiple Sclerosis
Abstract
Background: Since 2010, 27 mixed-treatment comparisons (MTCs) of disease-modifying therapies (DMTs) for multiple sclerosis have been published. However, there has been continued evolution in the field of MTCs. Additionally, limitations in methodological approach and reporting transparency, even in the most recent publications, makes interpretation and comparison of existing studies difficult.
Objectives: The objectives of this study are twofold: (1) to estimate the efficacy and safety of DMTs at European Commission-approved doses compared with placebo in adults with relapsing-remitting multiple sclerosis (RRMS) using MTC, and (2) to identify and address methodological challenges when performing MTC in RRMS, thereby creating a baseline for comparisons with future treatments.
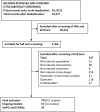
Methods: Searches were completed in 14 databases, including MEDLINE, Embase, CENTRAL, CDSR and DARE, from inception to June 2018 to identify published or unpublished prospective, randomised controlled trials of all European Union-approved DMTs or DMTs expected to be approved in the near future in RRMS or rapidly-evolving severe RRMS. No language or date restrictions were applied. Studies were included in the MTC if they were judged to have sufficiently similar characteristics, based on the following: patient age; proportion of male participants; Expanded Disability Status Scale (EDSS) score; duration of disease; number of relapses prior to enrolment and proportion of previously treated patients. Background information from the included studies, as well as effect size and confidence intervals (where relevant) of defined outcomes were extracted. Reporting of the MTC was consistent with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) network meta-analysis guidelines.
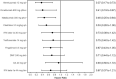
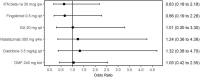
Results: In total, 33 studies were included in the MTC. Annualised relapse rate (ARR 28 trials) was significantly reduced in all treatments compared with placebo. Alemtuzumab had the highest probability (63%) of being the most effective treatment in terms of ARR compared with placebo (rate ratio [RR] 0.28, 95% credible interval [CrI] 0.21-0.38), followed by natalizumab (30% probability; RR 0.32, 95% CrI 0.23-0.43). The risk of 3- and 6-month confirmed disability progression (CDP3M, 13 trials; CDP6M, 14 trials) were similar; CDP6M was significantly reduced for alemtuzumab (hazard ratio [HR] 0.365; 95% CrI 0.165-0.725), ocrelizumab (HR 0.405, 95% CrI 0.188-0.853) and natalizumab (HR 0.459, 95% CrI 0.252-0.840) relative to placebo. There were no significant differences in the odds of serious adverse events (SAEs, 6 trials) between any treatment and placebo. The results of the MTC were limited by the lack of studies reporting direct comparisons between the included treatments and by heterogeneous reporting of key outcome data.
Conclusions: Meta-analyses confirmed the benefit of all DMTs in terms of relapse rate compared with placebo with a comparable rate of SAEs for the DMTs that could be included in the network. The rigor and transparency of reporting in this study provide a benchmark for comparisons with future new agents.
Keywords: Disease-modifying therapies; Meta-analysis; Mixed treatment comparison; Multiple sclerosis; Relapsing–remitting multiple sclerosis.
Figures



References
-
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials. London: National Institute for Health and Care Excellence (NICE); 2014. http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD4-Inconsistency.fina.... Accessed 03 Feb 2020. - PubMed
-
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. - PubMed
-
- Ades A, Caldwell DM, Reken S, Welton NJ, Sutton AJ, Dias S. NICE DSU technical Support document 7: evidence synthesis of treatment efficacy in decision making: a reviewers checklist. London: National Institute for Health and Care Excellence (NICE); 2012. http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD7-reviewer-checklist.... Accessed 03 Feb 2020. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

