Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds
- PMID: 32990218
- PMCID: PMC7524549
- DOI: 10.7554/eLife.60066
Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds
Abstract
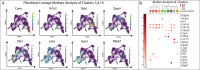
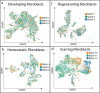
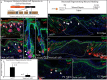
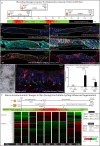
Scars are a serious health concern for burn victims and individuals with skin conditions associated with wound healing. Here, we identify regenerative factors in neonatal murine skin that transforms adult skin to regenerate instead of only repairing wounds with a scar, without perturbing development and homeostasis. Using scRNA-seq to probe unsorted cells from regenerating, scarring, homeostatic, and developing skin, we identified neonatal papillary fibroblasts that form a transient regenerative cell type that promotes healthy skin regeneration in young skin. These fibroblasts are defined by the expression of a canonical Wnt transcription factor Lef1 and using gain- and loss of function genetic mouse models, we demonstrate that Lef1 expression in fibroblasts primes the adult skin macroenvironment to enhance skin repair, including regeneration of hair follicles with arrector pili muscles in healed wounds. Finally, we share our genomic data in an interactive, searchable companion website (https://skinregeneration.org/). Together, these data and resources provide a platform to leverage the regenerative abilities of neonatal skin to develop clinically tractable solutions that promote the regeneration of adult tissue.
Keywords: Lef1; dermal papilla; fibroblast heterogeneity; mouse; regeneration; regenerative medicine; stem cells; wound healing.
© 2020, Phan et al.
Conflict of interest statement
QP, GF, LS, GH, BW, ID, RD No competing interests declared
Figures






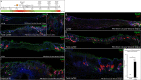
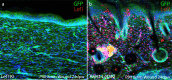
References
-
- Abbasi S, Sinha S, Labit E, Rosin NL, Yoon G, Rahmani W, Jaffer A, Sharma N, Hagner A, Shah P, Arora R, Yoon J, Islam A, Uchida A, Chang CK, Stratton JA, Scott RW, Rossi FMV, Underhill TM, Biernaskie J. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell. 2020;27:396–412. doi: 10.1016/j.stem.2020.07.008. - DOI - PubMed
-
- Adam RC, Yang H, Ge Y, Lien W-H, Wang P, Zhao Y, Polak L, Levorse J, Baksh SC, Zheng D, Fuchs E. Temporal layering of signaling effectors drives chromatin remodeling during hair follicle stem cell lineage progression. Cell Stem Cell. 2018;22:398–413. doi: 10.1016/j.stem.2017.12.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

