Metabolic dysfunction in human skin: Restoration of mitochondrial integrity and metabolic output by nicotinamide (niacinamide) in primary dermal fibroblasts from older aged donors
- PMID: 32990346
- PMCID: PMC7576238
- DOI: 10.1111/acel.13248
Metabolic dysfunction in human skin: Restoration of mitochondrial integrity and metabolic output by nicotinamide (niacinamide) in primary dermal fibroblasts from older aged donors
Abstract
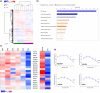
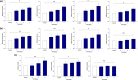
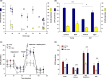
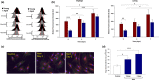
Alterations in metabolism in skin are accelerated by environmental stressors such as solar radiation, leading to premature aging. The impact of aging on mitochondria is of interest given their critical role for metabolic output and the finding that environmental stressors cause lowered energy output, particularly in fibroblasts where damage accumulates. To better understand these metabolic changes with aging, we performed an in-depth profiling of the expression patterns of dermal genes in face, forearm, and buttock biopsies from females of 20-70 years of age that encode for all subunits comprising complexes I-V of the mitochondrial electron transport chain. This complements previous preliminary analyses of these changes. "Oxidative phosphorylation" was the top canonical pathway associated with aging in the face, and genes encoding for numerous subunits had decreased expression patterns with age. Investigations on fibroblasts from older aged donors also showed decreased gene expression of numerous subunits from complexes I-V, oxidative phosphorylation rates, spare respiratory capacity, and mitochondrial number and membrane potential compared to younger cells. Treatment of older fibroblasts with nicotinamide (Nam) restored these measures to younger cell levels. Nam increased complexes I, IV, and V activity and gene expression of representative subunits. Elevated mt-Keima staining suggests a possible mechanism of action for these restorative effects via mitophagy. Nam also improved mitochondrial number and membrane potential in younger fibroblasts. These findings show there are significant changes in mitochondrial functionality with aging and that Nam treatment can restore bioenergetic efficiency and capacity in older fibroblasts with an amplifying effect in younger cells.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
J.E.O., J.D.S., B.B.J., M.R.B., and H.A.R. are full‐time employees of The Procter & Gamble Company (Cincinnati, OH, USA). All other authors declare no conflicts of interest.
Figures

Similar articles
-
Nicotinamide preferentially protects glycolysis in dermal fibroblasts under oxidative stress conditions.Br J Dermatol. 2013 Jul;169 Suppl 2:15-24. doi: 10.1111/bjd.12365. Br J Dermatol. 2013. PMID: 23786616
-
The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging.DNA Repair (Amst). 2014 Nov;23:59-63. doi: 10.1016/j.dnarep.2014.04.005. Epub 2014 Apr 30. DNA Repair (Amst). 2014. PMID: 24794404 Review.
-
Inadequate mito-biogenesis in primary dermal fibroblasts from old humans is associated with impairment of PGC1A-independent stimulation.Exp Gerontol. 2014 Aug;56:59-68. doi: 10.1016/j.exger.2014.03.017. Epub 2014 Mar 31. Exp Gerontol. 2014. PMID: 24699405
-
Modulation of Mitochondrial Membrane Potential and ROS Generation by Nicotinamide in a Manner Independent of SIRT1 and Mitophagy.Mol Cells. 2017 Jul 31;40(7):503-514. doi: 10.14348/molcells.2017.0081. Epub 2017 Jul 24. Mol Cells. 2017. PMID: 28736426 Free PMC article.
-
Respiratory function decline and DNA mutation in mitochondria, oxidative stress and altered gene expression during aging.Chang Gung Med J. 2009 Mar-Apr;32(2):113-32. Chang Gung Med J. 2009. PMID: 19403001 Review.
Cited by
-
Maintenance of Chronological Aging Features in Culture of Normal Human Dermal Fibroblasts from Old Donors.Cells. 2022 Mar 2;11(5):858. doi: 10.3390/cells11050858. Cells. 2022. PMID: 35269480 Free PMC article.
-
Mitoribosomal Deregulation Drives Senescence via TPP1-Mediated Telomere Deprotection.Cells. 2022 Jun 30;11(13):2079. doi: 10.3390/cells11132079. Cells. 2022. PMID: 35805162 Free PMC article.
-
Novel Approach to Skin Anti-Aging: Boosting Pharmacological Effects of Exogenous Nicotinamide Adenine Dinucleotide (NAD+) by Synergistic Inhibition of CD38 Expression.Cells. 2024 Oct 30;13(21):1799. doi: 10.3390/cells13211799. Cells. 2024. PMID: 39513906 Free PMC article.
-
Mitochondrial dynamics and metabolism across skin cells: implications for skin homeostasis and aging.Front Physiol. 2023 Nov 15;14:1284410. doi: 10.3389/fphys.2023.1284410. eCollection 2023. Front Physiol. 2023. PMID: 38046945 Free PMC article. Review.
-
Carnitine acetyltransferase deficiency mediates mitochondrial dysfunction-induced cellular senescence in dermal fibroblasts.Aging Cell. 2023 Nov;22(11):e14000. doi: 10.1111/acel.14000. Epub 2023 Oct 13. Aging Cell. 2023. PMID: 37828898 Free PMC article.
References
-
- Baracca, A. , Solaini, G. , Sgarbi, G. , Lenaz, G. , Baruzzi, A. , Schapira, A. , Martinuzzi, A. , & Carelli, V. (2020). Severe impairment of complex I‐driven adenosine triphosphate synthesis in leber hereditary optic neuropathy cybrids. Archives of Neurology, 62, 730–736. - PubMed
-
- Birch‐Machin, M. A. , & Bowman, A. (2016). Oxidative stress and ageing. British Journal of Dermatology, 175(Suppl 2), 26–29. - PubMed
-
- Birch‐Machin, M. A. , Briggs, H. L. , Saborido, A. A. , Bindoff, L. A. , & Turnbull, D. M. (1994). An evaluation of the measurement of the activities of complexes I‐IV in the respiratory chain of human skeletal muscle mitochondria. Biochemical Medicine and Metabolic Biology, 51(1), 35–42. - PubMed
-
- Birch‐Machin, M. A. , & Turnbull, D. M. (2001). Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biology, 65, 97–117. - PubMed
-
- Bowman, A. , & Birch‐Machin, M. A. (2016). Age‐dependent decrease of mitochondrial Complex II activity in human skin fibroblasts. Journal of Investigative Dermatology, 136(5), 912–919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

