Brain immune cells undergo cGAS/STING-dependent apoptosis during herpes simplex virus type 1 infection to limit type I IFN production
- PMID: 32990676
- PMCID: PMC7773356
- DOI: 10.1172/JCI136824
Brain immune cells undergo cGAS/STING-dependent apoptosis during herpes simplex virus type 1 infection to limit type I IFN production
Abstract
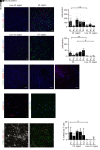
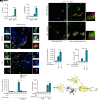
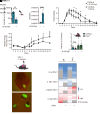
Protection of the brain from viral infections involves the type I IFN (IFN-I) system, defects in which render humans susceptible to herpes simplex encephalitis (HSE). However, excessive cerebral IFN-I levels lead to pathologies, suggesting the need for tight regulation of responses. Based on data from mouse models, human HSE cases, and primary cell culture systems, we showed that microglia and other immune cells undergo apoptosis in the HSV-1-infected brain through a mechanism dependent on the cyclic GMP-AMP synthase/stimulator of interferon genes (cGAS/STING) pathway, but independent of IFN-I. HSV-1 infection of microglia induced cGAS-dependent apoptosis at high viral doses, whereas lower viral doses led to IFN-I responses. Importantly, inhibition of caspase activity prevented microglial cell death and augmented IFN-I responses. Accordingly, HSV-1-infected organotypic brain slices or mice treated with a caspase inhibitor exhibited lower viral load and an improved infection outcome. Collectively, we identify an activation-induced apoptosis program in brain immune cells that downmodulates local immune responses.
Keywords: Apoptosis pathways; Immunology; Infectious disease; Innate immunity; Neurological disorders.
Conflict of interest statement
Figures






References
-
- Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM eds. Fields Virology. Lippincott, Williams & Wilkins; 2001:2461–2509.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

