Interchangeability of Biosimilars: What Level of Clinical Evidence is Needed to Support the Interchangeability Designation in the United States?
- PMID: 32990892
- PMCID: PMC7669758
- DOI: 10.1007/s40259-020-00446-7
Interchangeability of Biosimilars: What Level of Clinical Evidence is Needed to Support the Interchangeability Designation in the United States?
Abstract
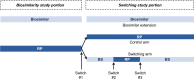
A biosimilar is a biologic drug that is "highly similar to a reference (originator) product, with no clinically meaningful differences between the two products in safety, purity, and potency". Regulatory approval of a biosimilar is based on analytical, structural, and functional comparisons with the reference product, comparative nonclinical (in vivo) studies, clinical pharmacokinetics and/or pharmacodynamics, and immunogenicity. In addition, comparative clinical efficacy and safety assessments are usually conducted and, taken together, comprise the "totality of the evidence" supporting biosimilarity. For a biosimilar to meet the additional designation of interchangeability in the United States (US), the applicant must demonstrate that the biological drug can be expected to produce the "same clinical result as the reference product in any given patient" and "if the biological drug is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between the use of the biological drug and the reference product is no greater than the risk of using the reference product without such alternation or switch". The challenges faced in conducting clinical studies to support a designation of interchangeability, as defined in the final interchangeability guidance from the US Food and Drug Administration, are considered. Potential alternative approaches to generating adequate and sufficient clinical data to support a designation of interchangeability are also presented.
Figures

References
-
- US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry. 2015. https://www.fda.gov/media/82647/download. Accessed 11 Nov 2019.
-
- US Food and Drug Administration. Considerations in demonstrating interchangeability with a reference product. Guidance for industry. 2019. https://www.fda.gov/media/124907/download. Accessed 21 May 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

