Real-World Clinical Experience With Idebenone in the Treatment of Leber Hereditary Optic Neuropathy
- PMID: 32991388
- PMCID: PMC7657145
- DOI: 10.1097/WNO.0000000000001023
Real-World Clinical Experience With Idebenone in the Treatment of Leber Hereditary Optic Neuropathy
Abstract
Background: Leber hereditary optic neuropathy (LHON) leads to bilateral central vision loss. In a clinical trial setting, idebenone has been shown to be safe and to provide a trend toward improved visual acuity, but long-term evidence of effectiveness in real-world clinical practice is sparse.
Methods: Open-label, multicenter, retrospective, noncontrolled analysis of long-term visual acuity and safety in 111 LHON patients treated with idebenone (900 mg/day) in an expanded access program. Eligible patients had a confirmed mitochondrial DNA mutation and had experienced the onset of symptoms (most recent eye) within 1 year before enrollment. Data on visual acuity and adverse events were collected as per normal clinical practice. Efficacy was assessed as the proportion of patients with either a clinically relevant recovery (CRR) or a clinically relevant stabilization (CRS) of visual acuity. In the case of CRR, time to and magnitude of recovery over the course of time were also assessed.
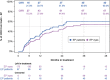
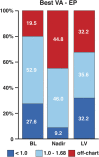
Results: At time of analysis, 87 patients had provided longitudinal efficacy data. Average treatment duration was 25.6 months. CRR was observed in 46.0% of patients. Analysis of treatment effect by duration showed that the proportion of patients with recovery and the magnitude of recovery increased with treatment duration. Average gain in best-corrected visual acuity for responders was 0.72 logarithm of the minimal angle of resolution (logMAR), equivalent to more than 7 lines on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. Furthermore, 50% of patients who had a visual acuity below 1.0 logMAR in at least one eye at initiation of treatment successfully maintained their vision below this threshold by last observation. Idebenone was well tolerated, with most adverse events classified as minor.
Conclusions: These data demonstrate the benefit of idebenone treatment in recovering lost vision and maintaining good residual vision in a real-world setting. Together, these findings indicate that idebenone treatment should be initiated early and be maintained more than 24 months to maximize efficacy. Safety results were consistent with the known safety profile of idebenone.
Conflict of interest statement
M. Silva, G. Metz, and X. Llòria are regular employees of Santhera. T. Klopstock has received research support, consultancy fees, speaker honoraria, and travel funds from GenSight Biologics and Santhera Pharmaceuticals, unrelated to this paper. The remaining authors report no conflicts of interest.
Figures

Comment in
-
Assessing the Treatment Effect of Idebenone in Leber Hereditary Optic Neuropathy Requires Appropriate Study Designs.J Neuroophthalmol. 2023 Sep 1;43(3):e95-e96. doi: 10.1097/WNO.0000000000001317. Epub 2021 Jul 26. J Neuroophthalmol. 2023. PMID: 34314394 No abstract available.
-
Real-World Clinical Experience With Idebenone in the Treatment of Leber Hereditary Optic Neuropathy-Response to Dr. Finsterer's Letter.J Neuroophthalmol. 2023 Sep 1;43(3):e96. doi: 10.1097/WNO.0000000000001318. Epub 2021 Aug 6. J Neuroophthalmol. 2023. PMID: 34387631 No abstract available.
Dataset use reported in
-
Kaplan-Meier Statistics to Estimate Treatment Success.J Neuroophthalmol. 2023 Dec 1;43(4):e360-e361. doi: 10.1097/WNO.0000000000001627. Epub 2022 Jun 14. J Neuroophthalmol. 2023. PMID: 36166805 No abstract available.
References
-
- Gueven N. Optic neurodegeneration: time to act. Biol Med. 2014;01.
-
- Carelli V, Carbonelli M, de Coo IF, Kawasaki A, Klopstock T, Lagrèze WA, La Morgia C, Newman NJ, Orssaud C, Pott JWR, Sadun AA, van Everdingen J, Vignal-Clermont C, Votruba M, Yu-Wai-Man P, Barboni P. International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy. J Neuroophthalmol. 2017;37:371–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

