Histamine H2 receptor negatively regulates oligodendrocyte differentiation in neonatal hypoxic-ischemic white matter injury
- PMID: 32991666
- PMCID: PMC7527977
- DOI: 10.1084/jem.20191365
Histamine H2 receptor negatively regulates oligodendrocyte differentiation in neonatal hypoxic-ischemic white matter injury
Abstract
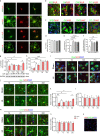
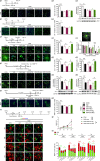
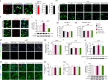
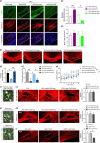
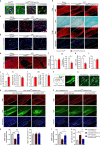
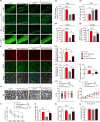
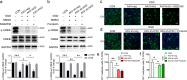
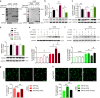
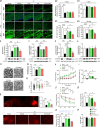
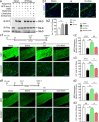
Neonatal hypoxic-ischemic encephalopathy (HIE) with the pathological characteristic of white matter injury often leads to lifelong cognitive and neurobehavioral dysfunction, but relevant therapies to promote remyelination are still unavailable. We found that histamine H2 receptor (H2R) negatively regulated the oligodendrocyte differentiation rate without affecting the oligodendrocytes at the oligodendrocyte precursor cell stage or mature stage following oxygen-glucose deprivation in vitro. Notably, selective deletion of the H2R gene (Hrh2) in differentiating oligodendrocytes (Hrh2fl/fl;CNPase-Cre) improved their differentiation, remyelination, and functional recovery following neonatal hypoxia-ischemia in mice. The regulation of oligodendrocyte differentiation by H2R is mediated by binding with Axin2, which leads to up-regulation of the Wnt/β-catenin signaling pathway. Furthermore, H2R antagonists also promoted oligodendrocyte differentiation and remyelination and the recovery of cognition and motor functions following neonatal hypoxia-ischemia. Thus, histamine H2R in oligodendrocytes could serve as a novel and effective therapeutic target for the retard of oligodendrocyte differentiation and remyelination following neonatal hypoxia-ischemia. The H2R antagonists may have potential therapeutic value for neonatal HIE.
© 2020 Jiang et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

References
-
- Billiards S.S., Haynes R.L., Folkerth R.D., Borenstein N.S., Trachtenberg F.L., Rowitch D.H., Ligon K.L., Volpe J.J., and Kinney H.C.. 2008. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 18:153–163. 10.1111/j.1750-3639.2007.00107.x - DOI - PMC - PubMed
-
- Blakemore W.F., and Franklin R.. 2008. Remyelination in experimental models of toxin-induced demyelination. In Current Topics in Microbiology and Immunology. Rodriguez M., editor. Springer-Verlag Berlin, Berlin: pp. 193–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

