Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma
- PMID: 32992341
- PMCID: PMC7955404
- DOI: 10.1182/blood.2020007400
Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma
Abstract
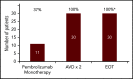
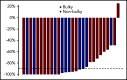
Pembrolizumab, a humanized IgG4 monoclonal antibody targeting programmed death-1 protein, has demonstrated efficacy in relapsed/refractory classical Hodgkin lymphoma (cHL). To assess the complete metabolic response (CMR) rate and safety of pembrolizumab monotherapy in newly diagnosed cHL, we conducted a multicenter, single-arm, phase 2 investigator-initiated trial of sequential pembrolizumab and doxorubicin, vinblastine, and dacarbazine (AVD) chemotherapy. Patients ≥18 years of age with untreated, early, unfavorable, or advanced-stage disease were eligible for treatment. Thirty patients (early unfavorable stage, n = 12; advanced stage, n = 18) were treated with 3 cycles of pembrolizumab monotherapy followed by AVD for 4 to 6 cycles, depending on stage and bulk. Twelve had either large mediastinal masses or bulky disease (>10 cm). After pembrolizumab monotherapy, 11 patients (37%) demonstrated CMRs, and an additional 7 of 28 (25%) patients with quantifiable positron emission tomography computed tomography scans had >90% reduction in metabolic tumor volume. All patients achieved CMR after 2 cycles of AVD and maintained their responses at the end of treatment. With a median follow-up of 22.5 months (range, 14.2-30.6) there were no changes in therapy, progressions, or deaths. No patients received consolidation radiotherapy, including those with bulky disease. Therapy was well tolerated. The most common immune-related adverse events were grade 1 rash (n = 6) and grade 2 infusion reactions (n = 4). One patient had reversible grade 4 transaminitis and a second had reversible Bell's palsy. Brief pembrolizumab monotherapy followed by AVD was both highly effective and safe in patients with newly diagnosed cHL, including those with bulky disease. This trial was registered at www.clinicaltrials.gov as #NCT03226249.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.B.A. has received honoraria from the advisory boards of Imbrium and Bayer and fees from the speakers bureaus of Curio Science and Purdue Pharma LP, outside the submitted work. A.M.E. has received grants from ORIEN and Tesaro and other support from Seattle Genetics, MorphoSys, Mylteni, Epizyme, Novartis, Pharmacyclics, Research to Practice, Physician Education Resource, Cota, and OncLive, outside the submitted work. R.H.A. reports consulting or advisory roles with Genentech/Roche, Portola Pharmaceuticals, Sanofi, Seattle Genetics, Takeda; grants from Celgene, Forty Seven, Inc, Genentech/Roche, Janssen Pharmaceutical, Kura, Merck, Millennium Pharmacyclics, Regeneron, and Seattle Genetics, outside the submitted work. B.P. has received grants and personal fees from Takeda and grants and personal fees from Seattle Genetics, outside the submitted work. R.K. has received rants and personal fees from Gilead and Kite; grants and personal fees from BMS, Celgene, and Juno; grants from Takeda; and personal fees from BeiGene, AstraZeneca, and Karyopharm, outside the submitted work; J.N.W. reports an honorarium for serving on an advisory board for Merck and for Karyopharm, and that her spouse serves on data and safety monitoring boards for Novartis, Ariad/Takeda, and Epizyme and as a consultant to Novartis, CVS/Caremark, Fly Pharma, Astra Zeneca, and Amgen. The remaining authors declare no competing financial interests.
Figures



Comment in
-
PD-1 blockade for untreated Hodgkin lymphoma.Blood. 2021 Mar 11;137(10):1271-1272. doi: 10.1182/blood.2020009281. Blood. 2021. PMID: 33704392 No abstract available.
References
-
- André MPE, Girinsky T, Federico M, et al. Early Positron Emission Tomography Response-Adapted Treatment in Stage I and II Hodgkin Lymphoma: Final Results of the Randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2017;35(16):1786-1794. - PubMed
-
- Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684-691. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

