Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes
- PMID: 32992479
- PMCID: PMC7600916
- DOI: 10.3390/nu12102954
Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes
Abstract
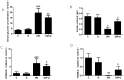
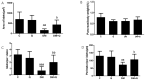
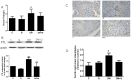
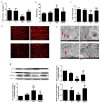
(1) Background: Pancreatic iron deposition has been found in the progression of type 2 diabetes (T2DM); however, whether ferroptosis contributes to the dysfunction of pancreatic β cells (PBC) remains enigmatic. Moreover, the potential protective effect of quercetin is also elusive; (2) Methods: T2DM mice model was established by multiple low dose streptozocin (STZ) injection, after which quercetin was intervened for 4 months; (3) Results: Substantially normalized glucose tolerance, diabetic symptoms, homeostasis model assessment for insulin resistance (HOMA-IR), and homeostasis model assessment for β cell (HOMA-β) index in comparison with the findings of T2DM control. Distorted pancreatic islets and especially shrunken mitochondria with cristae loss in PBC were observed in T2DM mice, which was ameliorated by quercetin. Meanwhile, quercetin lowered the iron level particularly in the islet in T2DM mice. In spite of compensatory xCT up-regulation, T2DM molding depleted glutathione (GSH), down-regulated glutathione peroxidase 4 (GPX4), and induced oxidative stress in pancreatic tissue, which was abolished partially by quercetin. More importantly, insulin secretion was worsened by ferroptosis-inducing erastin or RAS-selective lethal compounds 3 (RSL-3). Quercetin, ferroptosis inhibitor ferrostatin-1 and iron-chelating deferoxamine, rescued cell viability when cells were challenged with high-glucose; (4) Conclusions: Our findings identify that ferroptosis contributes to the PBC loss and dysfunction. Quercetin exerts beneficial effects on T2DM potentially by inhibiting pancreatic iron deposition and PBC ferroptosis, highlighting promising control strategies of T2DM by quercetin.
Keywords: ferroptosis; iron overload; lipid peroxidation; quercetin; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- International Diabetes Federation . IDF Diabetes Atlas. 9th ed. International Diabetes Federation; Brussels, Belgium: 2019.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

