Enzymes Involved in the Biosynthesis of Arginine from Ornithine in Maritime Pine (Pinus pinaster Ait.)
- PMID: 32992504
- PMCID: PMC7601404
- DOI: 10.3390/plants9101271
Enzymes Involved in the Biosynthesis of Arginine from Ornithine in Maritime Pine (Pinus pinaster Ait.)
Abstract
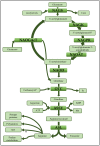
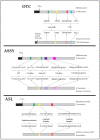
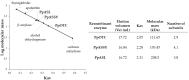
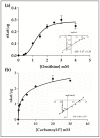
The amino acids arginine and ornithine are the precursors of a wide range of nitrogenous compounds in all living organisms. The metabolic conversion of ornithine into arginine is catalyzed by the sequential activities of the enzymes ornithine transcarbamylase (OTC), argininosuccinate synthetase (ASSY) and argininosuccinate lyase (ASL). Because of their roles in the urea cycle, these enzymes have been purified and extensively studied in a variety of animal models. However, the available information about their molecular characteristics, kinetic and regulatory properties is relatively limited in plants. In conifers, arginine plays a crucial role as a main constituent of N-rich storage proteins in seeds and serves as the main source of nitrogen for the germinating embryo. In this work, recombinant PpOTC, PpASSY and PpASL enzymes from maritime pine (Pinus pinaster Ait.) were produced in Escherichia coli to enable study of their molecular and kinetics properties. The results reported here provide a molecular basis for the regulation of arginine and ornithine metabolism at the enzymatic level, suggesting that the reaction catalyzed by OTC is a regulatory target in the homeostasis of ornithine pools that can be either used for the biosynthesis of arginine in plastids or other nitrogenous compounds in the cytosol.
Keywords: Pinus; amino acid biosynthesis; arginine; biochemical regulation; conifers; enzyme kinetics; nitrogen metabolism; ornithine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
The role of arginine metabolic pathway during embryogenesis and germination in maritime pine (Pinus pinaster Ait.).Tree Physiol. 2018 Mar 1;38(3):471-484. doi: 10.1093/treephys/tpx133. Tree Physiol. 2018. PMID: 29112758
-
Structural and Functional Characteristics of Two Molecular Variants of the Nitrogen Sensor PII in Maritime Pine.Front Plant Sci. 2020 Jun 16;11:823. doi: 10.3389/fpls.2020.00823. eCollection 2020. Front Plant Sci. 2020. PMID: 32612622 Free PMC article.
-
Argininosuccinate synthetase and argininosuccinate lyase: two ornithine cycle enzymes from Agaricus bisporus.Mycol Res. 2007 Apr;111(Pt 4):493-502. doi: 10.1016/j.mycres.2007.01.016. Epub 2007 Feb 8. Mycol Res. 2007. PMID: 17512708
-
Ornithine Transcarbamylase - From Structure to Metabolism: An Update.Front Physiol. 2021 Oct 1;12:748249. doi: 10.3389/fphys.2021.748249. eCollection 2021. Front Physiol. 2021. PMID: 34658931 Free PMC article. Review.
-
Genotype-Phenotype Correlations in Ornithine Transcarbamylase Deficiency: A Mutation Update.J Genet Genomics. 2015 May 20;42(5):181-94. doi: 10.1016/j.jgg.2015.04.003. Epub 2015 May 19. J Genet Genomics. 2015. PMID: 26059767 Free PMC article. Review.
Cited by
-
The effect of high-intensity interval training on type 2 diabetic muscle: A metabolomics-based study.Heliyon. 2024 Jul 20;10(15):e34917. doi: 10.1016/j.heliyon.2024.e34917. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170342 Free PMC article.
-
Evolutionary implications of C2 photosynthesis: how complex biochemical trade-offs may limit C4 evolution.J Exp Bot. 2023 Feb 5;74(3):707-722. doi: 10.1093/jxb/erac465. J Exp Bot. 2023. PMID: 36437625 Free PMC article.
-
Arginine inhibits the arginine biosynthesis rate-limiting enzyme and leads to the accumulation of intracellular aspartate in Synechocystis sp. PCC 6803.Plant Mol Biol. 2024 Mar 13;114(2):27. doi: 10.1007/s11103-024-01416-1. Plant Mol Biol. 2024. PMID: 38478146 Free PMC article.
-
An insight into role of amino acids as antioxidants via NRF2 activation.Amino Acids. 2024 Mar 20;56(1):23. doi: 10.1007/s00726-024-03384-8. Amino Acids. 2024. PMID: 38506925 Free PMC article. Review.
-
Special Issue Editorial: Plant Nitrogen Assimilation and Metabolism.Plants (Basel). 2021 Jun 23;10(7):1278. doi: 10.3390/plants10071278. Plants (Basel). 2021. PMID: 34201753 Free PMC article.
References
-
- Cañas R.A., de la Torre F., Pascual M.B., Ávila C., Cánovas F.M. Nitrogen economy and nitrogen environmental interactions in conifers. Agronomy. 2016;6:26. doi: 10.3390/agronomy6020026. - DOI
-
- Li G., Coleman G.D. Nitrogen storage and cycling in trees. Adv. Bot. Res. 2019;89:127–148. doi: 10.1016/bs.abr.2018.11.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

