Three-Dimensional Cell Culture Systems in Radiopharmaceutical Cancer Research
- PMID: 32993034
- PMCID: PMC7600608
- DOI: 10.3390/cancers12102765
Three-Dimensional Cell Culture Systems in Radiopharmaceutical Cancer Research
Abstract
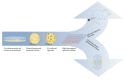
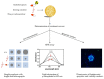
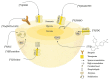
In preclinical cancer research, three-dimensional (3D) cell culture systems such as multicellular spheroids and organoids are becoming increasingly important. They provide valuable information before studies on animal models begin and, in some cases, are even suitable for reducing or replacing animal experiments. Furthermore, they recapitulate microtumors, metastases, and the tumor microenvironment much better than monolayer culture systems could. Three-dimensional models show higher structural complexity and diverse cell interactions while reflecting (patho)physiological phenomena such as oxygen and nutrient gradients in the course of their growth or development. These interactions and properties are of great importance for understanding the pathophysiological importance of stromal cells and the extracellular matrix for tumor progression, treatment response, or resistance mechanisms of solid tumors. Special emphasis is placed on co-cultivation with tumor-associated cells, which further increases the predictive value of 3D models, e.g., for drug development. The aim of this overview is to shed light on selected 3D models and their advantages and disadvantages, especially from the radiopharmacist's point of view with focus on the suitability of 3D models for the radiopharmacological characterization of novel radiotracers and radiotherapeutics. Special attention is paid to pancreatic ductal adenocarcinoma (PDAC) as a predestined target for the development of new radionuclide-based theranostics.
Keywords: 3D model; co-culture; organoids; pancreatic cancer; radiotherapeutics; radiotracer; spheroids; stromal cells; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no influence on the conception of the review, on the interpretation of literature data, on the conclusions drawn, or in the decision to publish this review.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

