Repurposing existing drugs for COVID-19: an endocrinology perspective
- PMID: 32993622
- PMCID: PMC7523486
- DOI: 10.1186/s12902-020-00626-0
Repurposing existing drugs for COVID-19: an endocrinology perspective
Abstract
Background: Coronavirus Disease 2019 (COVID-19) is a multi-systemic infection caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), that has become a pandemic. Although its prevailing symptoms include anosmia, ageusia, dry couch, fever, shortness of brief, arthralgia, myalgia, and fatigue, regional and methodological assessments vary, leading to heterogeneous clinical descriptions of COVID-19. Aging, uncontrolled diabetes, hypertension, obesity, and exposure to androgens have been correlated with worse prognosis in COVID-19. Abnormalities in the renin-angiotensin-aldosterone system (RAAS), angiotensin-converting enzyme-2 (ACE2) and the androgen-driven transmembrane serine protease 2 (TMPRSS2) have been elicited as key modulators of SARS-CoV-2.
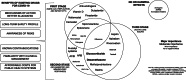
Main text: While safe and effective therapies for COVID-19 lack, the current moment of pandemic urges for therapeutic options. Existing drugs should be preferred over novel ones for clinical testing due to four inherent characteristics: 1. Well-established long-term safety profile, known risks and contraindications; 2. More accurate predictions of clinical effects; 3. Familiarity of clinical management; and 4. Affordable costs for public health systems. In the context of the key modulators of SARS-CoV-2 infectivity, endocrine targets have become central as candidates for COVID-19. The only endocrine or endocrine-related drug class with already existing emerging evidence for COVID-19 is the glucocorticoids, particularly for the use of dexamethasone for severely affected patients. Other drugs that are more likely to present clinical effects despite the lack of specific evidence for COVID-19 include anti-androgens (spironolactone, eplerenone, finasteride and dutasteride), statins, N-acetyl cysteine (NAC), ACE inhibitors (ACEi), angiotensin receptor blockers (ARB), and direct TMPRSS-2 inhibitors (nafamostat and camostat). Several other candidates show less consistent plausibility. In common, except for dexamethasone, all candidates have no evidence for COVID-19, and clinical trials are needed.
Conclusion: While dexamethasone may reduce mortality in severely ill patients with COVID-19, in the absence of evidence of any specific drug for mild-to-moderate COVID-19, researchers should consider testing existing drugs due to their favorable safety, familiarity, and cost profile. However, except for dexamethasone in severe COVID-19, drug treatments for COVID-19 patients must be restricted to clinical research studies until efficacy has been extensively proven, with favorable outcomes in terms of reduction in hospitalization, mechanical ventilation, and death.
Keywords: ACE2; COVID-19; Pandemic; SARS-CoV-2; TMPRSS2.
Conflict of interest statement
The authors declare no financial competing interests.
Figures
References
-
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published online ahead of print, 2020 Mar 11] [published correction appears in Lancet. 2020 Mar 12;:]. Lancet. 2020;S0140-6736(20)30566-3. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous