Role of sex and high-fat diet in metabolic and hypothalamic disturbances in the 3xTg-AD mouse model of Alzheimer's disease
- PMID: 32993686
- PMCID: PMC7526387
- DOI: 10.1186/s12974-020-01956-5
Role of sex and high-fat diet in metabolic and hypothalamic disturbances in the 3xTg-AD mouse model of Alzheimer's disease
Abstract
Background: Hypothalamic dysfunction occurs early in the clinical course of Alzheimer's disease (AD), likely contributing to disturbances in feeding behavior and metabolic function that are often observed years prior to the onset of cognitive symptoms. Late-life weight loss and low BMI are associated with increased risk of dementia and faster progression of disease. However, high-fat diet and metabolic disease (e.g., obesity, type 2 diabetes), particularly in mid-life, are associated with increased risk of AD, as well as exacerbated AD pathology and behavioral deficits in animal models. In the current study, we explored possible relationships between hypothalamic function, diet/metabolic status, and AD. Considering the sex bias in AD, with women representing two-thirds of AD patients, we sought to determine whether these relationships vary by sex.
Methods: WT and 3xTg-AD male and female mice were fed a control (10% fat) or high-fat (HF 60% fat) diet from ~ 3-7 months of age, then tested for metabolic and hypothalamic disturbances.
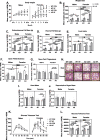
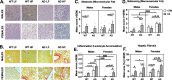
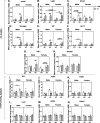
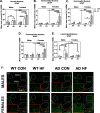
Results: On control diet, male 3xTg-AD mice displayed decreased body weight, reduced fat mass, hypoleptinemia, and mild systemic inflammation, as well as increased expression of gliosis- and inflammation-related genes in the hypothalamus (Iba1, GFAP, TNF-α, IL-1β). In contrast, female 3xTg-AD mice on control diet displayed metabolic disturbances opposite that of 3xTg-AD males (increased body and fat mass, impaired glucose tolerance). HF diet resulted in expected metabolic alterations across groups (increased body and fat mass; glucose intolerance; increased plasma insulin and leptin, decreased ghrelin; nonalcoholic fatty liver disease-related pathology). HF diet resulted in the greatest weight gain, adiposity, and glucose intolerance in 3xTg-AD females, which were associated with markedly increased hypothalamic expression of GFAP and IL-1β, as well as GFAP labeling in several hypothalamic nuclei that regulate energy balance. In contrast, HF diet increased diabetes markers and systemic inflammation preferentially in AD males but did not exacerbate hypothalamic inflammation in this group.
Conclusions: These findings provide further evidence for the roles of hypothalamic and metabolic dysfunction in AD, which in the 3xTg-AD mouse model appears to be dependent on both sex and diet.
Keywords: Alzheimer’s disease; Diabetes; High-fat diet; Hypothalamus; Inflammation; Metabolic; Obesity; Sex.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

