Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation
- PMID: 32993751
- PMCID: PMC7523258
- DOI: 10.1186/s13054-020-03306-6
Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation
Abstract
Background: The severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is extremely variable, ranging from asymptomatic patients to those who develop severe acute respiratory distress syndrome (ARDS). As for now, there are still no really effective therapies for coronavirus disease 2019 (COVID-19). Some evidences suggest that tocilizumab (TCZ) may avoid the progression of severe COVID-19. The aim of this retrospective case-control study was to analyze the efficacy and safety of TCZ in patients with COVID-19 ARDS undergoing noninvasive mechanical ventilation (NIV).
Methods: Seventy-nine consecutive patients with severe COVID-19 pneumonia and worsening acute respiratory failure (ARF) were admitted to the Pulmonology Unit of Azienda USL of Reggio Emilia-IRCCS. All patients were inflamed (elevated CRP and IL-6 levels) and received NIV at admission according to the presence of a pO2/FiO2 ratio ≤ 200 mmHg. The possibility of being treated with TCZ depended on the drug availability. The primary outcome was the in-hospital mortality rate. A secondary composite outcome of worsening was represented by the patients who died in the pulmonology unit or were intubated.
Results: Out of 79 patients, 41 were treated with TCZ. Twenty-eight patients received intravenous (IV) TCZ and 13 patients received subcutaneous (SC) TCZ. In-hospital overall mortality rate was 38% (30/79 patients). The probabilities of dying and being intubated during the follow-up using Kaplan-Meier method were significantly lower in total patients treated with TCZ compared to those of patients not treated with TCZ (log-rank p value = 0.006 and 0.036, respectively). However, using Cox multivariate analyses adjusted for age and Charlson comorbidity index only the association with the reduced risk of being intubated or dying maintained the significance (HR 0.44, 95%CI 0.22-0.89, p = 0.022). Two patients treated with TCZ developed cavitating lung lesions during the follow-up.
Conclusions: This study shows that TCZ treatment may be effective in COVID-19 patients with severe respiratory impairment receiving NIV. More data on safety are required. Randomized controlled trials are needed to confirm these results.
Keywords: Acute respiratory distress syndrome; COVID-19; Noninvasive ventilation; SARS-CoV-2; Tocilizumab.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures
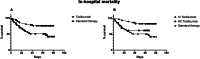

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

