Fibroblasts direct differentiation of human breast epithelial progenitors
- PMID: 32993755
- PMCID: PMC7526135
- DOI: 10.1186/s13058-020-01344-0
Fibroblasts direct differentiation of human breast epithelial progenitors
Abstract
Background: Breast cancer arises within specific regions in the human breast referred to as the terminal duct lobular units (TDLUs). These are relatively dynamic structures characterized by sex hormone driven cyclic epithelial turnover. TDLUs consist of unique parenchymal entities embedded within a fibroblast-rich lobular stroma. Here, we established and characterized a new human breast lobular fibroblast cell line against its interlobular counterpart with a view to assessing the role of region-specific stromal cues in the control of TDLU dynamics.
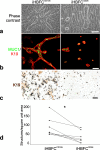
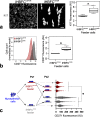
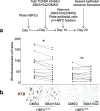
Methods: Primary lobular and interlobular fibroblasts were transduced to express human telomerase reverse transcriptase (hTERT). Differentiation of the established cell lines along lobular and interlobular pathways was determined by immunocytochemical staining and genome-wide RNA sequencing. Their functional properties were further characterized by analysis of mesenchymal stem cell (MSC) differentiation repertoire in culture and in vivo. The cells' physiological relevance for parenchymal differentiation was examined in heterotypic co-culture with fluorescence-activated cell sorting (FACS)-purified normal breast primary luminal or myoepithelial progenitors. The co-cultures were immunostained for quantitative assessment of epithelial branching morphogenesis, polarization, growth, and luminal epithelial maturation. In extension, myoepithelial progenitors were tested for luminal differentiation capacity in culture and in mouse xenografts. To unravel the significance of transforming growth factor-beta (TGF-β)-mediated crosstalk in TDLU-like morphogenesis and differentiation, fibroblasts were incubated with the TGF-β signaling inhibitor, SB431542, prior to heterotypic co-culture with luminal cells.
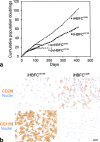
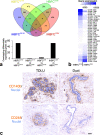
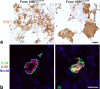
Results: hTERT immortalized fibroblast cell lines retained critical phenotypic traits in culture and linked to primary fibroblasts. Cell culture assays and transplantation to mice showed that the origin of fibroblasts determines TDLU-like and ductal-like differentiation of epithelial progenitors. Whereas lobular fibroblasts supported a high level of branching morphogenesis by luminal cells, interlobular fibroblasts supported ductal-like myoepithelial characteristics. TDLU-like morphogenesis, at least in part, relied on intact TGF-β signaling.
Conclusions: The significance of the most prominent cell type in normal breast stroma, the fibroblast, in directing epithelial differentiation is largely unknown. Through establishment of lobular and interlobular fibroblast cell lines, we here demonstrate that epithelial progenitors are submitted to stromal cues for site-specific differentiation. Our findings lend credence to considering stromal subtleties of crucial importance in the development of normal breast and, in turn, breast cancer.
Keywords: Breast; Differentiation; Epithelial progenitors; Fibroblast.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferrori SR, Herault Y, Pavlovic G, Ferguson-Smith AC, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. - DOI - PMC - PubMed
-
- Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55:231–273. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

