Empagliflozin and Cardiovascular and Kidney Outcomes across KDIGO Risk Categories: Post Hoc Analysis of a Randomized, Double-Blind, Placebo-Controlled, Multinational Trial
- PMID: 32994159
- PMCID: PMC7536760
- DOI: 10.2215/CJN.14901219
Empagliflozin and Cardiovascular and Kidney Outcomes across KDIGO Risk Categories: Post Hoc Analysis of a Randomized, Double-Blind, Placebo-Controlled, Multinational Trial
Abstract
Background and objectives: In the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG Outcome), empagliflozin, in addition to standard of care, significantly reduced risk of cardiovascular death by 38%, hospitalization for heart failure by 35%, and incident or worsening nephropathy by 39% compared with placebo in patients with type 2 diabetes and established cardiovascular disease. Using EMPA-REG Outcome data, we assessed whether the Kidney Disease Improving Global Outcomes (KDIGO) CKD classification had an influence on the treatment effect of empagliflozin.
Design, setting, participants, & measurements: Patients with type 2 diabetes, established atherosclerotic cardiovascular disease, and eGFR≥30 ml/min per 1.73 m2 at screening were randomized to receive empagliflozin 10 mg, empagliflozin 25 mg, or placebo once daily in addition to standard of care. Post hoc, we analyzed cardiovascular and kidney outcomes, and safety, using the two-dimensional KDIGO classification framework.
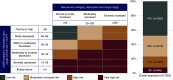
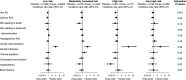
Results: Of 6952 patients with baseline eGFR and urinary albumin-creatinine ratio values, 47%, 29%, 15%, and 8% were classified into low, moderately increased, high, and very high KDIGO risk categories, respectively. Empagliflozin showed consistent risk reductions across KDIGO categories for cardiovascular outcomes (P values for treatment by subgroup interactions ranged from 0.26 to 0.85) and kidney outcomes (P values for treatment by subgroup interactions ranged from 0.16 to 0.60). In all KDIGO risk categories, placebo and empagliflozin had similar adverse event rates, the notable exception being genital infection events, which were more common with empagliflozin for each category.
Conclusions: The observed effects of empagliflozin versus placebo on cardiovascular and kidney outcomes were consistent across the KDIGO risk categories, indicating that the effect of treatment benefit of empagliflozin was unaffected by baseline CKD status.
Clinical trial registry name and registration number: EMPA-REG OUTCOME, NCT01131676.
Keywords: KDIGO; SGLT2 inhibition; diabetic nephropathy; empagliflozin; glomerular filtration rate; kidney disease.
Copyright © 2020 by the American Society of Nephrology.
Figures





Comment in
-
SGLT2 Inhibitors across the Spectrum of Severity of CKD.Clin J Am Soc Nephrol. 2020 Oct 7;15(10):1386-1388. doi: 10.2215/CJN.13430820. Epub 2020 Sep 29. Clin J Am Soc Nephrol. 2020. PMID: 33001842 Free PMC article. No abstract available.
References
-
- American Diabetes Association : Standards of medical care in diabetes—2014. Diabetes Care 37[Suppl 1]: S14–S80, 2014. - PubMed
-
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013
-
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003. - PubMed
-
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

