Strategies to Promote ResiliencY (SPRY): a randomised embedded multifactorial adaptative platform (REMAP) clinical trial protocol to study interventions to improve recovery after surgery in high-risk patients
- PMID: 32994242
- PMCID: PMC7526307
- DOI: 10.1136/bmjopen-2020-037690
Strategies to Promote ResiliencY (SPRY): a randomised embedded multifactorial adaptative platform (REMAP) clinical trial protocol to study interventions to improve recovery after surgery in high-risk patients
Abstract
Introduction: As the population ages, there is interest in strategies to promote resiliency, especially for frail patients at risk of its complications. The physiological stress of surgery in high-risk individuals has been proposed both as an important cause of accelerated age-related decline in health and as a model testing the effectiveness of strategies to improve resiliency to age-related health decline. We describe a randomised, embedded, multifactorial, adaptative platform (REMAP) trial to investigate multiple perioperative interventions, the first of which is metformin and selected for its anti-inflammatory and anti-ageing properties beyond its traditional blood glucose control features.
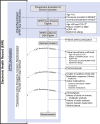
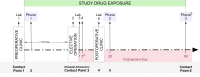
Methods and analysis: Within a multihospital, single healthcare system, the Core Protocol for Strategies to Promote ResiliencY (SPRY) will be embedded within both the electronic health record (EHR) and the healthcare culture generating a continuously self-learning healthcare system. Embedding reduces the administrative burden of a traditional trial while accessing and rapidly analysing routine patient care EHR data. SPRY-Metformin is a placebo-controlled trial and is the first SPRY domain evaluating the effectiveness of three metformin dosages across three preoperative durations within a heterogeneous set of major surgical procedures. The primary outcome is 90-day hospital-free days. Bayesian posterior probabilities guide interim decision-making with predefined rules to determine stopping for futility or superior dosing selection. Using response adaptative randomisation, a maximum of 2500 patients allows 77%-92% power, detecting >15% primary outcome improvement. Secondary outcomes include mortality, readmission and postoperative complications. A subset of patients will be selected for substudies evaluating the microbiome, cognition, postoperative delirium and strength.
Ethics and dissemination: The Core Protocol of SPRY REMAP and associated SPRY-Metformin Domain-Specific Appendix have been ethically approved by the Institutional Review Board and are publicly registered. Results will be publicly available to healthcare providers, patients and trial participants following achieving predetermined platform conclusions.
Trial registration number: NCT03861767.
Keywords: adult surgery; clinical trials; information management; surgery.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
REMAP Periop: a randomised, embedded, multifactorial adaptive platform trial protocol for perioperative medicine to determine the optimal enhanced recovery pathway components in complex abdominal surgery patients within a US healthcare system.BMJ Open. 2023 Dec 28;13(12):e078711. doi: 10.1136/bmjopen-2023-078711. BMJ Open. 2023. PMID: 38154902 Free PMC article.
-
The UPMC OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization.Trials. 2021 May 25;22(1):363. doi: 10.1186/s13063-021-05316-3. Trials. 2021. PMID: 34034784 Free PMC article.
-
ChemoPROphyLaxIs with hydroxychloroquine For covId-19 infeCtious disease (PROLIFIC) to prevent covid-19 infection in frontline healthcare workers: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jul 2;21(1):604. doi: 10.1186/s13063-020-04543-4. Trials. 2020. PMID: 32616067 Free PMC article.
-
Interleukin-6 blocking agents for treating COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD013881. doi: 10.1002/14651858.CD013881. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jun 1;6:CD013881. doi: 10.1002/14651858.CD013881.pub2. PMID: 33734435 Free PMC article. Updated.
-
Adult patient access to electronic health records.Cochrane Database Syst Rev. 2021 Feb 26;2(2):CD012707. doi: 10.1002/14651858.CD012707.pub2. Cochrane Database Syst Rev. 2021. PMID: 33634854 Free PMC article.
Cited by
-
Strategies to Promote Resiliency: A Randomized Embedded Multifactorial Adaptative Platform (REMAP) Clinical Trial to Study Interventions to Improve Recovery After Surgery in High-Risk Patients.Ann Surg Open. 2025 Apr 2;6(2):e566. doi: 10.1097/AS9.0000000000000566. eCollection 2025 Jun. Ann Surg Open. 2025. PMID: 40557356 Free PMC article.
-
Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19 : A Cohort Study.Ann Intern Med. 2023 Apr;176(4):496-504. doi: 10.7326/M22-1286. Epub 2023 Apr 4. Ann Intern Med. 2023. PMID: 37011399 Free PMC article. Clinical Trial.
-
Evaluation of Bebtelovimab for Treatment of Covid-19 During the SARS-CoV-2 Omicron Variant Era.Open Forum Infect Dis. 2022 Oct 1;9(10):ofac517. doi: 10.1093/ofid/ofac517. eCollection 2022 Oct. Open Forum Infect Dis. 2022. PMID: 36324319 Free PMC article.
-
MetfOrmin BenefIts Lower Extremities with Intermittent Claudication (MOBILE IC): randomized clinical trial protocol.BMC Cardiovasc Disord. 2023 Jan 21;23(1):38. doi: 10.1186/s12872-023-03047-8. BMC Cardiovasc Disord. 2023. PMID: 36681798 Free PMC article.
-
The comparative effectiveness of COVID-19 monoclonal antibodies: A learning health system randomized clinical trial.Contemp Clin Trials. 2022 Aug;119:106822. doi: 10.1016/j.cct.2022.106822. Epub 2022 Jun 11. Contemp Clin Trials. 2022. PMID: 35697146 Free PMC article. Clinical Trial.
References
-
- 2017 National Population Projections Table United States census Bur, 2017.
-
- Walston J, Hadley EC, Ferrucci L, et al. . Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American geriatrics Society/National Institute on aging research conference on frailty in older adults. J Am Geriatr Soc 2006;54:991–1001. 10.1111/j.1532-5415.2006.00745.x - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
