Longitudinal exploration of cancer-related cognitive impairment in patients with newly diagnosed aggressive lymphoma: protocol for a feasibility study
- PMID: 32994248
- PMCID: PMC7526311
- DOI: 10.1136/bmjopen-2020-038312
Longitudinal exploration of cancer-related cognitive impairment in patients with newly diagnosed aggressive lymphoma: protocol for a feasibility study
Abstract
Introduction: Cancer-related cognitive impairment (CRCI) is a distressing and disabling side-effect of cancer treatments affecting up to 75% of patients. For some patients, their cognitive impairment may be transient, but for a subgroup, these symptoms can be long-standing and have a major impact on the quality of life. This paper describes the protocol for a study: (1) to assess the feasibility of collecting longitudinal data on cognition via self-report, neuropsychological testing, peripheral markers of inflammation and neuroimaging and (2) to explore and describe patterns of cancer-related cognitive impairment over the course of treatment and recovery in patients with newly diagnosed, aggressive lymphoma undergoing standard therapy with curative intent.
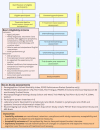
Methods and analysis: This is a prospective, longitudinal, feasibility study in which 30 newly diagnosed, treatment-naive patients with aggressive lymphoma will be recruited over a 12-month period. Patients will complete comprehensive assessments at three time points: baseline (time 1, pre-treatment) and two post-baseline follow-up assessments (time 2, mid-treatment and time 3, 6-8 weeks post-treatment completion). All patients will be assessed for self-reported cognitive difficulties and objective cognitive function using Stroop Colour and Word, Trail Making Test Part A and B, Hopkins Verbal Learning Test-Revised, Controlled Oral Word Association and Digit Span. Blood cell-based inflammatory markers and neuroimaging including a positron emission tomography (PET) with 18F-labelled fluoro-2-deoxyglucose (18F-FDG) and CT (18F-FDG-PET/CT) and a MRI will explore potential inflammatory and neuroanatomical or functional mechanisms and biomarkers related to CRCI. The primary intent of analysis will be to assess the feasibility of collecting longitudinal data on cognition using subjective reports and objective tasks from patients during treatment and recovery for lymphoma. These data will inform the design of a larger-scale investigation into the patterns of cognitive change over the course of treatment and recovery, adding to an underexplored area of cancer survivorship research.
Ethics and dissemination: Ethical approval has been granted by Austin Health Human Rights Ethics Committee (HREC) in Victoria Australia. Peer reviewed publications and conference presentations will report the findings of this novel study.
Trial registration number: Australian New Zealand Clinical Trials Registry (ACTRN12619001649101).
Keywords: adult neurology; delirium & cognitive disorders; lymphoma.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Web-based cognitive rehabilitation intervention for cancer-related cognitive impairment following chemotherapy for aggressive lymphoma: protocol for a randomised pilot trial.BMJ Open. 2024 Apr 22;14(4):e081084. doi: 10.1136/bmjopen-2023-081084. BMJ Open. 2024. PMID: 38653511 Free PMC article.
-
Cancer-related cognitive impairment in patients with newly diagnosed aggressive lymphoma undergoing standard chemotherapy: a longitudinal feasibility study.Support Care Cancer. 2022 Sep;30(9):7731-7743. doi: 10.1007/s00520-022-07153-9. Epub 2022 Jun 14. Support Care Cancer. 2022. PMID: 35699780 Free PMC article. Clinical Trial.
-
[18F]-fluoroethyl-L-tyrosine (FET) in glioblastoma (FIG) TROG 18.06 study: protocol for a prospective, multicentre PET/CT trial.BMJ Open. 2023 Aug 4;13(8):e071327. doi: 10.1136/bmjopen-2022-071327. BMJ Open. 2023. PMID: 37541751 Free PMC article.
-
Protocol for Care After Lymphoma (CALy) trial: a phase II pilot randomised controlled trial of a lymphoma nurse-led model of survivorship care.BMJ Open. 2016 May 18;6(5):e010817. doi: 10.1136/bmjopen-2015-010817. BMJ Open. 2016. PMID: 27194317 Free PMC article. Clinical Trial.
-
Neuroimaging based biotypes for precision diagnosis and prognosis in cancer-related cognitive impairment.Front Med (Lausanne). 2023 Aug 29;10:1199605. doi: 10.3389/fmed.2023.1199605. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37720513 Free PMC article. Review.
Cited by
-
Cancer-related cognitive impairment and wellbeing in patients with newly diagnosed aggressive lymphoma compared to population norms and healthy controls: an exploratory study.Support Care Cancer. 2024 Mar 21;32(4):238. doi: 10.1007/s00520-024-08441-2. Support Care Cancer. 2024. PMID: 38512692 Free PMC article.
-
Behavioral Characterizing of CD24 Knockout Mouse-Cognitive and Emotional Alternations.J Pers Med. 2021 Feb 6;11(2):105. doi: 10.3390/jpm11020105. J Pers Med. 2021. PMID: 33562144 Free PMC article.
-
Web-based cognitive rehabilitation intervention for cancer-related cognitive impairment following chemotherapy for aggressive lymphoma: protocol for a randomised pilot trial.BMJ Open. 2024 Apr 22;14(4):e081084. doi: 10.1136/bmjopen-2023-081084. BMJ Open. 2024. PMID: 38653511 Free PMC article.
-
Cancer-related cognitive impairment in patients with newly diagnosed aggressive lymphoma undergoing standard chemotherapy: a longitudinal feasibility study.Support Care Cancer. 2022 Sep;30(9):7731-7743. doi: 10.1007/s00520-022-07153-9. Epub 2022 Jun 14. Support Care Cancer. 2022. PMID: 35699780 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
